CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 3, April 2013
74
AFRICA
Four patients are currently being followed up, with ages of
0.7, 1.0, 3.0 and 3.7 years, respectively. In three of these, perma-
nent renal sequelae are present, namely renal atrophy, dysplastic
kidneys, asymmetric renal function and proteinuria. In one
of these, cortical atrophy with dysfunction of the kidney only
became apparent during follow up. Two still require antihyper-
tensive medication, but all have a normal shortening fraction.
Discussion
Over a four-year period in a busy neonatal unit in a referral
centre, only six patients with circulatory failure due to acute
hypertensive crisis were identified, indicating how rare this
condition is. These patients are usually critically ill and may die
if not diagnosed and treated promptly.
All our patients presented within two weeks of birth. This,
remarkably, resembles findings in the only other previously
published series where the median age at presentation was 7.2
days.
13
Similar to our findings, they also observed that the initial
clinical symptoms were mostly non-specific and related to
feeding and respiratory difficulties.
It is of clinical significance that all our patients presented
with some form of circulatory failure. Although most were
hypertensive (
n
=
3), two patients presented with hypotension and
shock and one was normotensive (Table 1). Hypertension due to
increased systemic vascular resistance only became apparent
after they were stabilised and resuscitated. The hypotension
was most likely caused by impaired left ventricular systolic
performance as confirmed by reduced fractional shortening.
In most of our patients, hypertrophy of the interventricular
septum and/or left ventricular posterior wall was evident.
This increase in left ventricular mass had also been reported
by Peterson.
13
Hypertension in our patients was most likely
of recent postnatal onset. We postulate that antenatal onset of
hypertension is unlikely, since one then would have expected
significant biventricular hypertrophy with significant pulmonary
hypertension, Such patients present with cyanosis due to atrial
right-to-left shunt.
14
The differential diagnosis of neonatal hypertension has been
extensively reviewed.
3
An important question to be answered
is what triggers these postnatal arterial hypertensive events?
Could it be related to the postnatal haemodynamic and humoral
changes which ‘relax’ the homeostatic vasomotor tone and elicit
an acute biochemical response? Alternatively, is it due to mostly
intrinsic renal abnormalities which then become manifest, or are
these events triggered by iatrogenic factors such as thrombi from
umbilical lines? Thromboembolic events related to umbilical
lines are acknowledged as the most common cause of clinical
hypertension in neonates. In this study, renal causes were
identified in two infants and thrombus in one. More studies are
needed to answer these questions.
Echocardiography is usually requested once an infant with
circulatory failure is admitted to the neonatal intensive care
unit. Faced with this clinical presentation, the demonstration
of hypocontractility would inevitably lead the cardiologist to
consider a differential diagnosis of myocarditis, cardiomyopathy,
coarctation of the aorta or coronary artery anomalies. Left
ventricular hypocontractility in the absence of hypertension
will be misleading in this case. However, careful analysis of the
abovementioned echocardiographic findings associated with
mild aortic regurgitation should alert the physician to consider
systemic hypertension as the probable underlying cause.
Mitral regurgitation is not unexpected in the presence of left
ventricular dysfunction and is frequently observed in association
with cardiomyopathy. However, aortic regurgitation is very
unusual in a supposedly normal heart, provided the valve is
structurally normal. Although the left heart was not dilated,
central aortic and mitral valve regurgitation were seen in the
majority of patients. We did not measure aortic diameter in this
study but Peterson and coworkers reported mild dilation in their
study, which they ascribed to the increased aortic distensability
Fig. 2. Thrombus in the left ventricular apex. Apical four-
chamber view demonstrating thrombus in the left ventric-
ular apex (arrow). LV, left ventricle; RV, right ventricle.
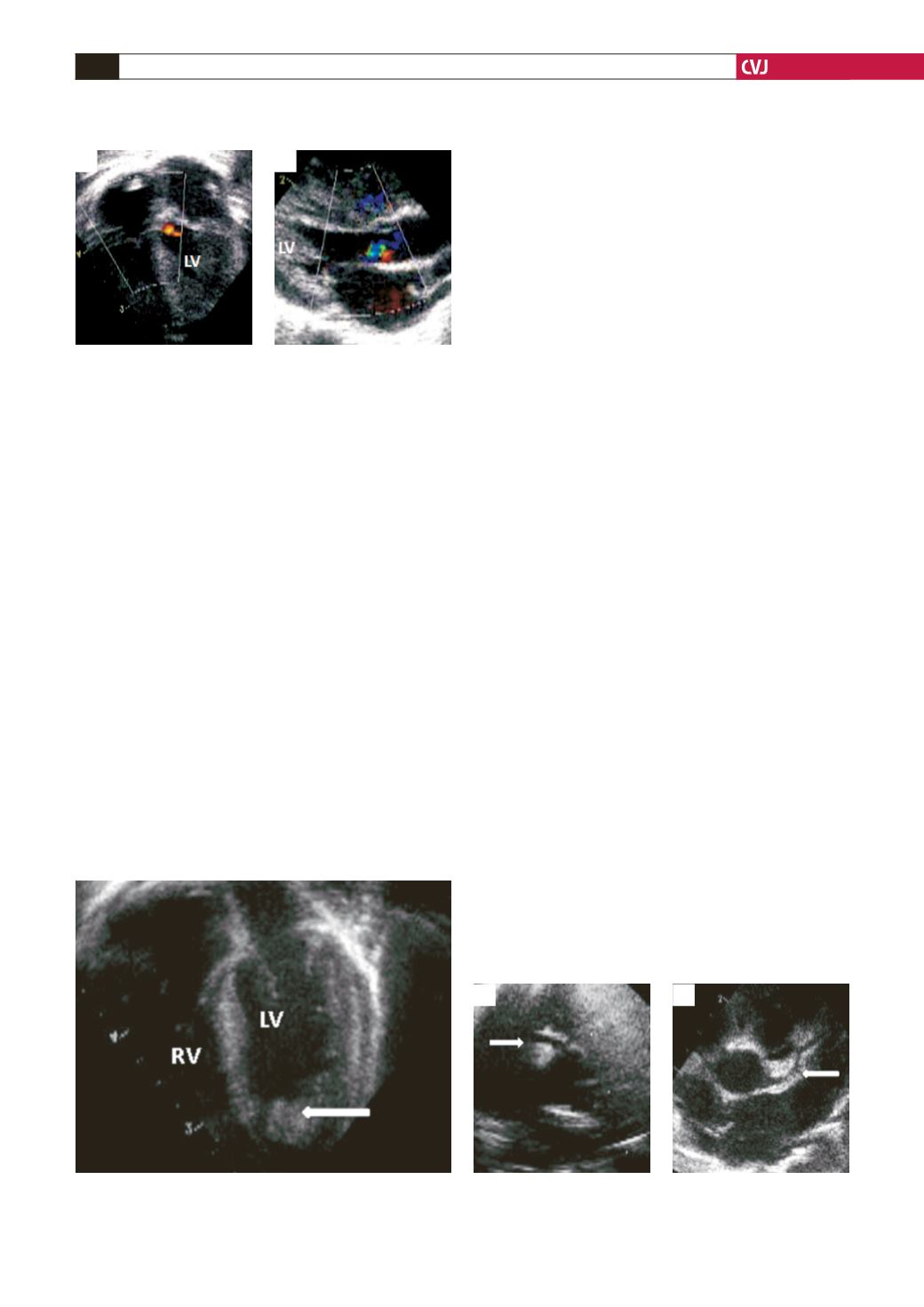
Fig. 1. Aortic regurgitation. Mild aortic regurgitation in
four-chamber view (A), and long-axial plane (B). LV, left
ventricle.
A
B
Fig. 3. Prominent coronary arteries. Short-axis image
with arrow indicating prominent right coronary artery (A),
and left coronary artery (B).
A
B


