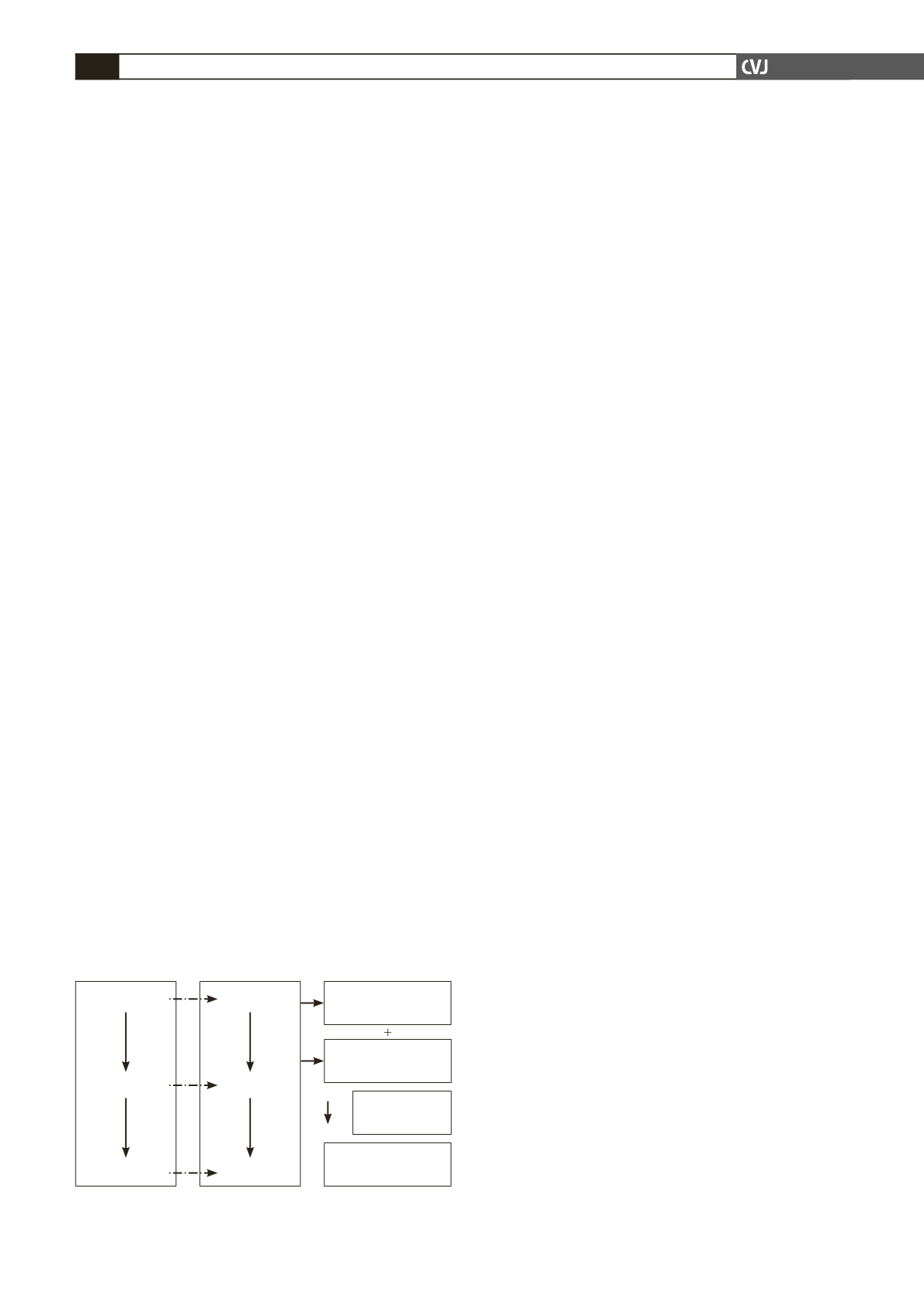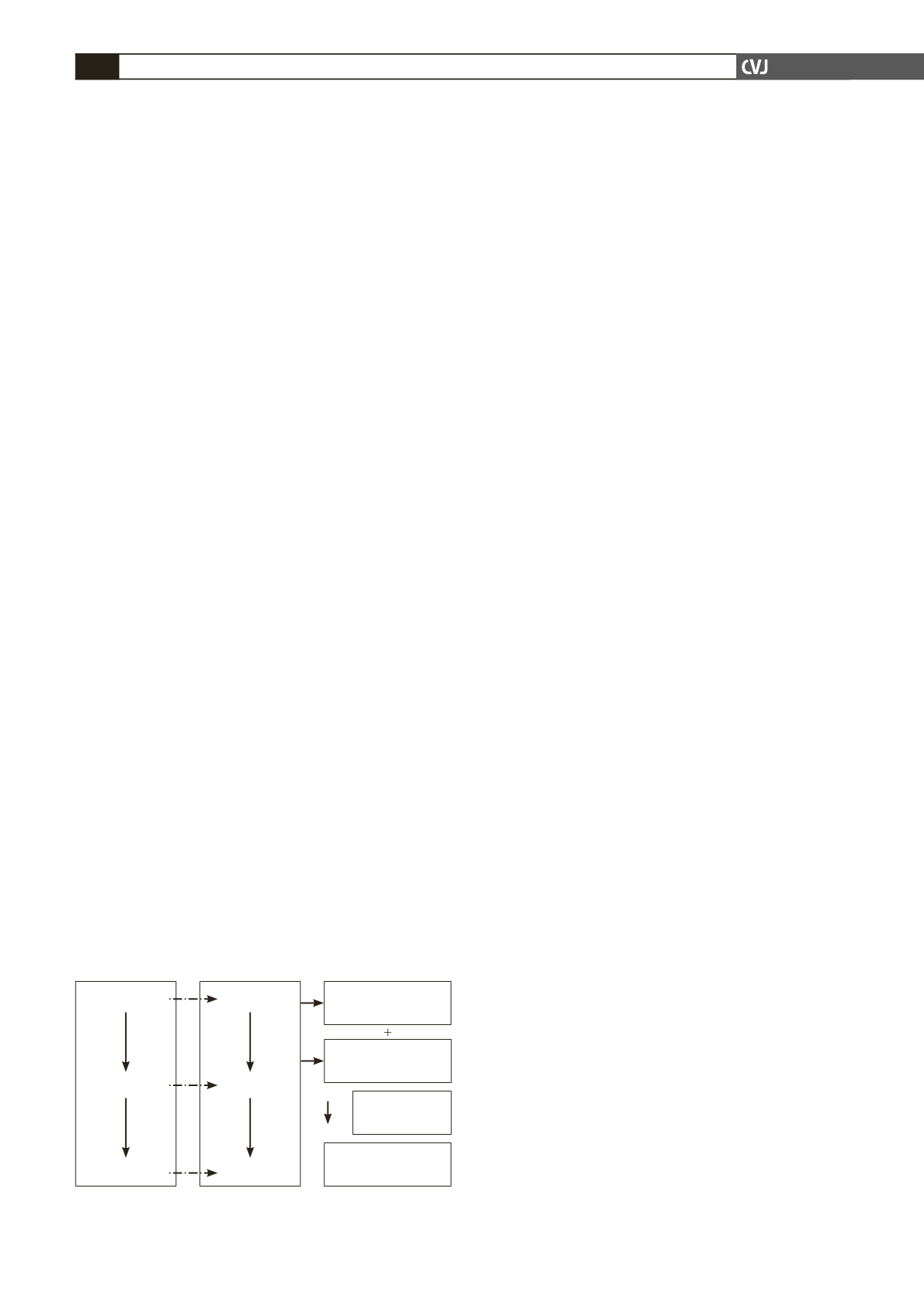
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 22, No 2, March/April 2011
80
AFRICA
≥
20/10 mmHg above the BP goals.
3,11
Additionally, fixed-drug
combinations offer several advantages over the separate prescrip-
tion of individual drugs.
11
The need for better control of arterial
hypertension justifies observational studies designed to better
understand the real-life management of hypertensive patients,
especially in Africa, where few hypertension studies have been
conducted.
The primary outcome of the ASTRAL study was to evalu-
ate the percentage of hypertensive patients achieving BP goals
after eight weeks of treatment with a fixed-dose combination of
ramipril/HCTZ, hence to generate data on the effectiveness of
the fixed-dose combination.
Ethics committee approval to undertake the survey was
obtained from hospitals in each country in accordance with
national and local regulations. Written, signed consent was
obtained from each of the patients included. The study was
conducted in accordance with the Helsinki II Declaration.
Methods
The study was a multi-centre, non-comparative, open-label,
observational study conducted across 36 centres in five sub-
Saharan African countries (Cameroon, Congo Brazzaville, DRC,
Madagascar and Nigeria). Men and women 18 years of age or
older with arterial hypertension uncontrolled by an ACE inhibi-
tor, a diuretic, or any other monotherapy or anti-hypertensive
combination not containing a diuretic in a fixed dose were
considered eligible for inclusion in the study. Exclusion criteria
were: known hypersensitivity to any component of the study
drug (Tritazide
®
), history of angio-oedema, patients with severe
renal or hepatic dysfunction, severe gout, secondary hyperten-
sion, and all pregnant or breastfeeding women. Although the
study was initially planned to include 460 consecutive hyperten-
sive patients from the 36 participating centres, only 449 patients
could be recruited and included to receive the study drug; 408
(90.9%) of these patients completed the study. The reasons for
non-completion of the study are summarised in Fig. 1.
Three visits were scheduled during the study. At visit one
(week 0), demographic data, history of co-morbidities, duration
of hypertension, blood pressures at first diagnosis of hyperten-
sion and previous anti-hypertensive treatment given, concomi-
tant medications, and CV risk factors were recorded. SBP and
DBP were measured and recorded in the seated position after
five minutes of rest in the medical office, using a validated
and calibrated electronic sphygmomanometer (OMRON M7,
OMRON, Tokyo, Japan) with an appropriate cuff size. A blood
pressure target was established, recommended lifestyle modifi-
cations were given to the patient and the ramipril/HCTZ daily
dose was prescribed, based exclusively on the decision of the
treating physician and in accordance with the prescribing infor-
mation in the summary of the product characteristics or package
insert. Body weight was recorded in kilograms and height was
measured in metres to determine the body mass index (BMI)
which was recorded as overweight if
≥
25 kg/m
2
and obese if
≥
30 kg/m
2
, according to the WHO guidelines.
13
At the follow-up visits at weeks four and eight (visits two
and three, respectively), BP was measured, adverse events were
assessed and treatment compliance rating was evaluated by
taking into account the unused number of tablets returned by the
patient on the follow-up visit and expressed as good (
<
four of
28 tablets returned), average (five to nine of 28 tablets returned)
or poor (
≥
10 of 28 tablets returned). The necessity for titration
to a higher dose of the study drug was evaluated in response to
BP goal.
The primary outcome of the ASTRAL study was to evalu-
ate the percentage of hypertensive patients achieving BP goals
after eight weeks of treatment with a fixed-dose combination of
ramipril/HCTZ. Blood pressure control was defined as SBP
<
140 mmHg and/or DBP
<
90 mmHg in non-diabetics and SBP
<
130 mmHg and/or DBP
<
80 mmHg in diabetic patients. The
secondary objectives included the assessment of variations in
SBP and DBP values between visits, compliance with treatment,
tolerance of the treatment and the factors influencing BP control.
Statistical analysis
The statistical analysis was performed by ClinStat CC,
Pretoria, South Africa. All analyses were carried out on SAS
(release 9.1.3). Descriptive statistical analyses were performed.
Qualitative variables were expressed as frequency counts and
percentages. Quantitative variables were summarised by mean
values, standard deviations, minimum and maximum values.
Within-subject comparisons (changes in blood pressures at
weeks four and eight relative to baseline) were tested for signifi-
cance by the paired
t
-test.
A logistic regression analysis was performed with goal
achievement as a dependent variable and the following predictor
variables: age, gender, history of diabetes, alcohol consumption,
renal dysfunction, diabetic nephropathy, microalbuminuria, cere-
brovascular accident and heart failure, BMI, concomitant treat-
ment with NSAIDs, daily dose of study drug and BP at inclusion.
In the logistic regression analysis, the following predictor vari-
ables were numeric: age, systolic blood pressure, diastolic blood
pressure and daily doses of study drugs. All the other predictor
variables were categorical. Adverse events were listed, indicating
intensity, relationship with the study drug, discontinuation in the
study, corrective treatment, outcome and seriousness. A
p-
value
<
0.05 was considered as significant.
Results
The baseline demographic and clinical features, co-morbidities
and associated CV risk factors of all the patients and for patients
from the five participating countries are shown in Tables 1 and
2. Four hundred and forty-nine hypertensive patients took part
in the study, with a mean age of 54.7
±
11.7 years (range 20–90
Fig. 1. Study time line and reasons for non-completion
of study.
Number lost to
follow up
=
24
Number of with-
drawals
=
15
Number of non-
completers
=
41
Week 0 (V1)
Week 4 (V2)
Week 8 (V3)
(
n
=
449)
(
n
=
414)
(
n
=
408)
+
Unknown
reasons
=
2