
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 2, March 2013
AFRICA
e5
The patient’s cranial CT scan was normal. There was no
bleeding or cerebral infarction. She had generalised tonic, clonic
seizures once due to post-CPR hypoxic encephalopathy. She had
a normal EEG and laboratory analyses, chest X-rays and 12-lead
ECG were normal. She had been in the anaesthesiology intensive
care unit for 15 days.
After the patient was discharged, she was referred to the
Cardiology Department. On physical examination she had
paraparesis and spasticity on the lower extremities. She had no
history of using psychotropic and class I anti-arrhythmic drugs,
cocaine or insulin. The 12-lead ECG showed a sinus rate of 96
beats/min with a suspicious type 1 pattern of Brugada syndrome.
The PR, QRS and corrected QT intervals were 142, 88 and 422
ms, respectively (Fig. 1).
Her blood pressure was 110/70 mmHg. Chest X-ray and
routine laboratory examinations, including serum electrolytes
were normal. The thyroid-function test at the time of evaluation
was also within normal limits.
Echocardiography showed normal cardiac structure and
function. There were no thoracic abnormalities on magnetic
resonance imagining (MRI). The 24- and 72-hour Holter ECG
showed a total of 25 premature ventricular, non-repetitive
complexes and the heart rate variability parameters were normal.
Blood pressure recorded over 24 hours was within normal
limits. Exercise-stress testing was negative for the induction of
either transient acute myocardial ischaemia or arrhythmias.
The patient was also sent to the neurology department. The
EEG was normal and cranial MRI was consistent with hypoxic
encephalopathy (small demyelinated areas) but showed no
haemorrhagia or infarction.
Informed consent was obtained from the patient and she
was taken to the electrophysiology laboratory with a suspected
diagnosis of BS. The drug-challenge test was performed by
intravenous administration of propafenone (2 mg/kg/10 min
= 90 mg for 10 min) but she had no documented spontaneous
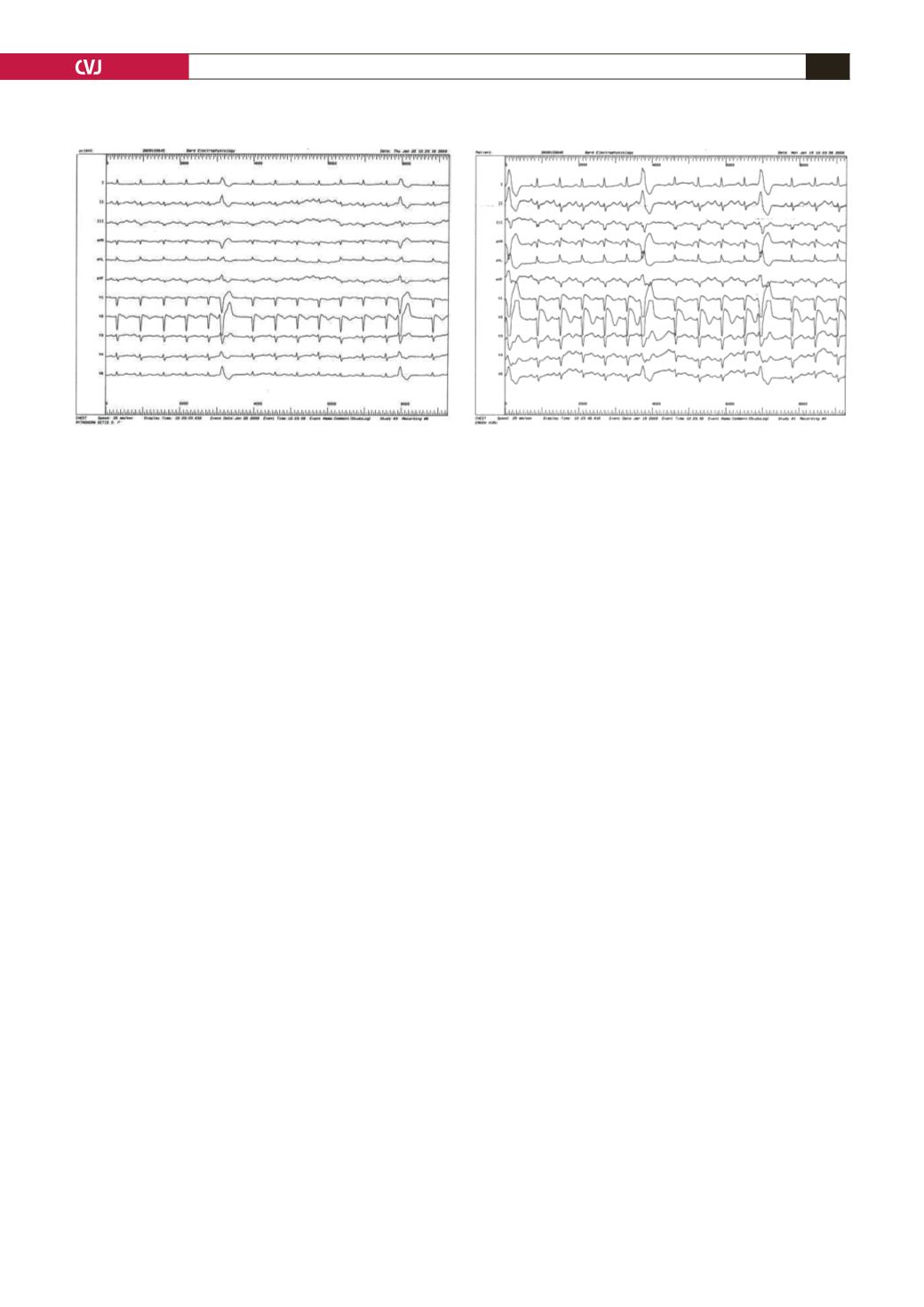
polymorphic ventricular tachycardia (VT). Minimal ST-segment
changes were seen compared with the basal ECG (Fig. 2) but it
was inadequate to describe BS because of minimal changes seen
in the patient’s basal ECGs from day to day.
The provocation test was performed one week later with
intravenous ajmaline (1.2 mg/kg/5 min), during which she was
continuously monitored (12-lead ECG and blood pressure). From
the first minute of the test a new gigantic coved type, down-
slopping ST-segment elevation and J waves were seen in leads
V1–3, and premature ventricular complexes (PVCs) developed
in pairs (Fig. 3). These changes reverted to the same as those in
the original ECG 25 minutes after administration of ajmaline.
The patient was treated with an implantable cardioverter
defibrillator (ICD) for cardiac arrest and VF. She was discharged
with a diagnosis of BS.
Discussion
Due to the prognostic implications for the affected individual,
it is important to recognise the suspect ECG pattern, which is
the cornerstone for diagnosis of BS.
7
However, there are certain
circumstances mimicking the Brugada ECG that should be
ruled out. Transient normalisation of the ECG signature of this
syndrome may lead to failure to recognise it. This could have
negative consequences on the management of these patients at
high risk for recurrence of lethal arrhythmias.
In this regard, inspection of previous ECGs and performance
of a baseline and follow-up ECG in all patients to whom class
I anti-arrhythmic drugs are prescribed, and carefully reviewing
it for the appearance of a typical pattern of right bundle branch
block and ST elevation seems good clinical practice, as it
could unmask the disease in patients with occult or borderline
ECG patterns. Furthermore, pharmacological interventions may
facilitate development of polymorphic VT/VF. The correct
diagnosis of a suspicious ECG pattern is of great importance in
saving a patient’s life and avoiding medico-legal consequences.
Suspicion of BS should therefore lead to the performance of a
pharmacological challenge.
A number of substances facilitate the elevation of the ST
segment by either reducing the inward sodium current or
increasing the outward potassiumcurrent.As the transient outward
current is better represented on the right than the left ventricular
epicardium, the transmural (epicardium–endocardium) voltage
gradient is amplified in the right precordial leads where the typical
ECG repolarisation abnormalities are usually displayed. Sodium
Fig. 2. Minimal ST changes after administration of
propafenone and rarely premature ventricular contrac-
tion (PVC).
Fig. 3. Newgiganticcoved-type, down-slopingST-segment
elevation and J waves seen in leads V1–3 and PVCs in
pairs after administration of ajmaline.



















