
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 6, November/December 2018
AFRICA
399
require laboratory evaluation and use of cardiovascular imaging
modalities that involve ionising radiation, which has been related
to teratogenesis, mutagenesis and childhood malignancy.
57
As
stated before, concerns related to safety of imaging tests must
always be balanced against the importance of accurate diagnosis
and thorough assessment of the pathological condition.
27
The indications for and limitations of the different diagnostic
procedures must be discussed, as well as their potentially harmful
effects during pregnancy, but if needed, chest radiography,
fluoroscopy, echocardiography and invasive angiography may
all be used. Echocardiography appears to be completely safe for
both the mother and foetus.
58
Counselling for pregnant women should be given according
to the CARPREG (CARdiac disease in PREGnancy) risk
score
47
or the modified World Health Organisation (WHO)
classification.
59
The fact that most women present to antenatal
clinics after 20 weeks of gestation has implications for their
functional assessment, and limits the option for termination
of pregnancy. In any case, care should be given to discussing
maternal and offspring risks, namely choices of anticoagulant
therapy, as well as risks of miscarriage, early delivery and small-
for-gestational-age babies. Complications such as heart failure
and valve thrombosis must also be discussed as they may occur
beyond the immediate delivery period. Finally, side effects of
common drugs need to be openly discussed.
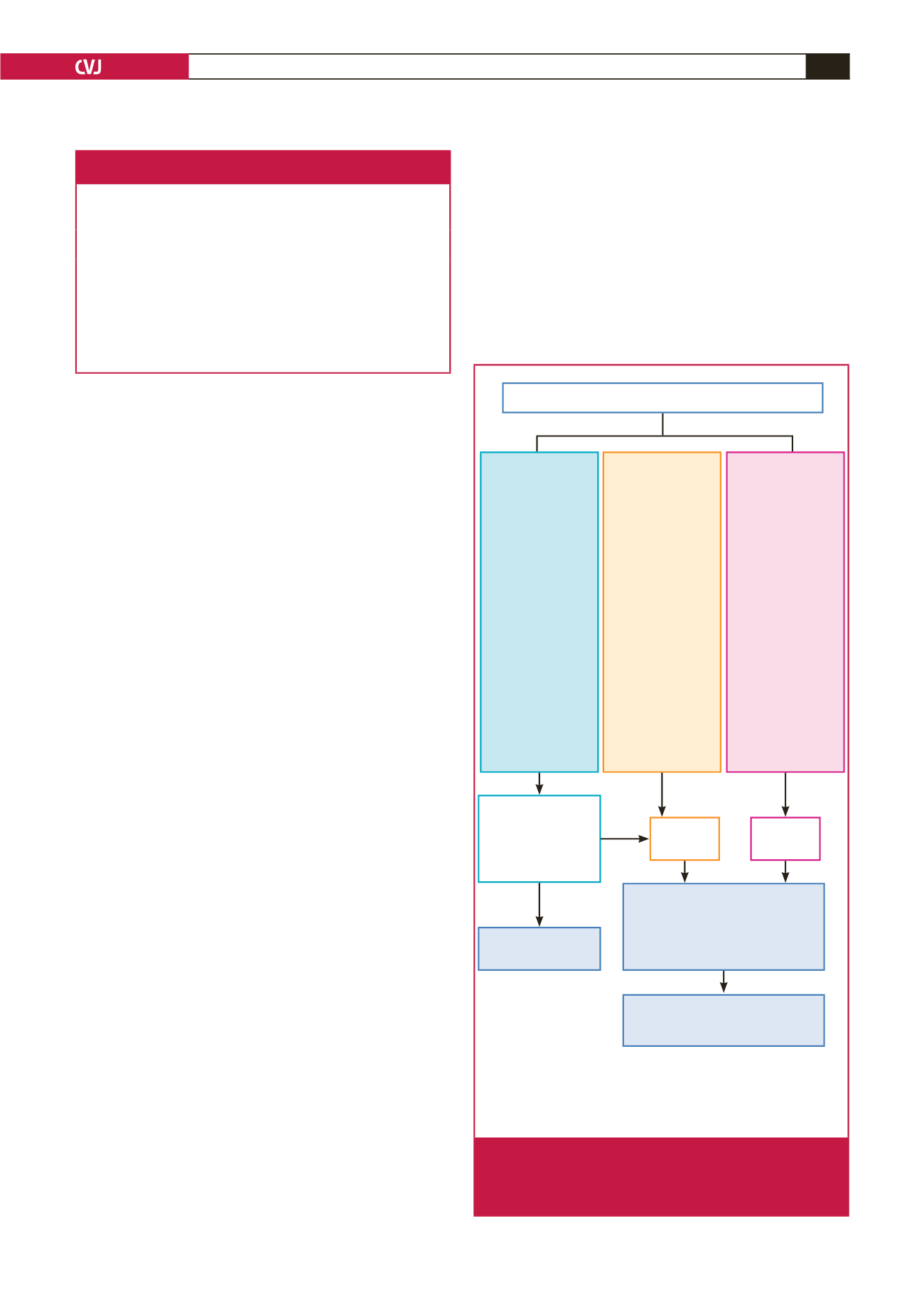
Algorithms for the identification and management of
pregnant patients with cardiovascular disease can help by
improving care, guiding screening of heart disease in all pregnant
women, and detecting those with RHD (Fig. 3). They should
distinguish between women with known or recently detected
cardiovascular disease who are controlled and should undergo
risk assessment, from those in heart failure needing immediate
ambulance transfer to a tertiary centre.
25
More importantly,
the algorithms must identify women at high risk who may need
careful monitoring beyond the usual peripartum period.
Labour and postpartum care
Induction, management of labour, delivery and postpartum
care require specific expertise and joint management by the
obstetrician, cardiologist and anesthaesiologist, preferably in
an experienced tertiary care centre. This is true for women with
native valve pathology but is more important in those with
prosthetic valves.
The treating specialist should prepare a detailed management
plan, considering access to care outside of normal working
hours. Management needs to be individualised due to the
complexity of cases but also due to lack of prospective data.
25
Based on consensus, the preferred mode of delivery is vaginal.
A delivery plan should be prepared until week 34, including
information on the timing of delivery (spontaneous/induced),
method of induction, use of general or regional anaesthesia, level
of monitoring, needs and details for post-partum monitoring,
and sub-acute bacterial endocarditis prophylaxis. In addition,
specific instructions for anticoagulation should be documented.
As recently summarised by Sliwa
et al
.,
25
delivery in
anticoagulated women with prosthetic valves needs to follow
a certain algorithm of care. At 36 weeks, most patients are
Table 1. Pre-conception evaluation in women with rheumatic heart
valve disease planning a pregnancy or assessment in early pregnancy
• Careful history, family history, and physical examination (if feasible, include
screening for connective tissue disorders)
• 12-lead electrocardiogram
• Echocardiogram including assessment of left- and right-ventricular and valve
function
• Laboratory assessment for risk factors: antistreptolysin O, erythosedimenta-
tion rate, haemoglobin, human immunodeficinecy virus test
• Exercise test to be considered for objective assessment of functional clas-
sification
• Careful counselling: maternal risks for complications and mortality, risk
of miscarriage, early delivery and small-for-gestational-age information on
choices of therapy (heparin vs vitamin K), when applicable, risk of foetal
congenital defect – inheritance risk
Primary and secondary care maternal facility
Modified WHO
classification I
• Previously diag-
nosed hyperten-
sion, diabetes,
morbid obesity
(BMI > 35 kg/m
2
)
• Successfully
repaired simple
lesions
• Uncomplicated,
small or mild mitral
valve prolapse,
pulmonary stenosis
• Palpitations – no
dizziness
Modified WHO
classification
III–IV
• Mechanical valve
and symptoms
• Complex congenital
or cyanotic heart
disease
• Pulmonary hyper-
tension any cause
• Previously diag-
nosed peripartum
cardiomyopathy
• Severe ventricular
impairment (EF <
45%, NYHA FC > II)
• Severe mitral
stenosis and aortic
stenosis
• Aortic dilataion
> 45 mm (bicuspid
AV, Marfan)
Modified WHO
classification II
• Unoperated ASD
and VSD
• Repaired tetralogy
of Fallot and coarc-
tation
• Arrythmias and
dizziness
• Mild left ventricular
impairment (EF >
45%, NYHA FC II)
due to newly diag-
nosed PPCM or HT
heart failure
• Previously diag-
nosed RHD with
murmurs and/or
recently assessed
asymptomatic
mechanical valve
Tertiary care
maternal facility
Tests: BP, ECG,
echocardiogram and
assess for murmurs
Non-urgent
referral
Urgent
referral
Joint cardiac–obstetric–
anaesthetic CDM team
Consulting with paediatric cardiologist,
endocrinologist, radiologist, HIV
specialist and others
Follow up with
maternity service
Postpartum referral to main cardiac
clinic, if indicated, for management
and possible cardiothoracic surgery
Abnormal
Normal
BMI: body mass index; ECG: electrocardiogram; ASD: atrial septal defect;
VSD: ventricular septal defect; EF: ejection fraction; NYHA FC: New York
Heart Association functional class; PPCM: peripartum cardiomyopathy;
HT: hypertension; AV: aortic valve
Fig. 3.
Referral algorithm recommended for screening and
management of women with suspected or known
cardiovascular disease at primary and secondary
level of care.

















