
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 1, January/February 2019
6
AFRICA
underlying causes, ACM, therapeutic interventions, and
morbidity and mortality rates. Thus follows a retrospective
review of all the children with RSH presenting to a large tertiary-
care hospital in southern Africa.
Methods
A retrospective review of children diagnosed with RSH at the
Chris Hani Baragwanath Academic Hospital (CHBAH) was
undertaken. The study spanned a 22-year period and patient
records were obtained from the electronic database of the
CHBAH cardiology department.
Data collected included age at diagnosis, gender, underlying
cause of the RSH, prevalence, ACM, extra-cardiac abnormalities,
situs, interventions and patient outcome at the time of the
study. We used chest roentgenogram, echocardiography,
electrocardiogram, cardiac catheterisation and foetal
ultrasonography either alone or in combination to diagnose the
RSH. ACM were grouped according to the diagnostic categories
described by DC Fyler and published in the New England
Regional Infant Cardiac Program in 1980.
15
Permission to conduct retrospective analyses was obtained
from the Human Research Ethics Committee of the University
of the Witwatersrand.
Statistical analysis
Descriptive statistical analysis was performed. The Chi-squared
test, unpaired Student’s
t
-test and Mann–Whitney
U
-test were
employed. A
p
-value
<
0.05 was used as the level of significance.
Data were collected and managed using REDCap (Research
Electronic Data Capture)
16
and were analysed using Microsoft
Excel and Graphpad Prism. REDCap
is a secure, web-based
application designed to support data capture for research studies,
hosted at the University of the Witwatersrand.
16
Results
There were 18 870 paediatric patients referred for cardiac
assessment between 1 January 1991 and 2 November 2012. One
hundred and eighty-six children were found to have RSH. This
comprised 1% of the total paediatric cardiology referrals seen
during the study period.
Of the 186 patients with RSH, 108 were diagnosed with
dextrocardia as the underlying cause. A further 76 patients
had dextroposition and only two had a confirmed diagnosis of
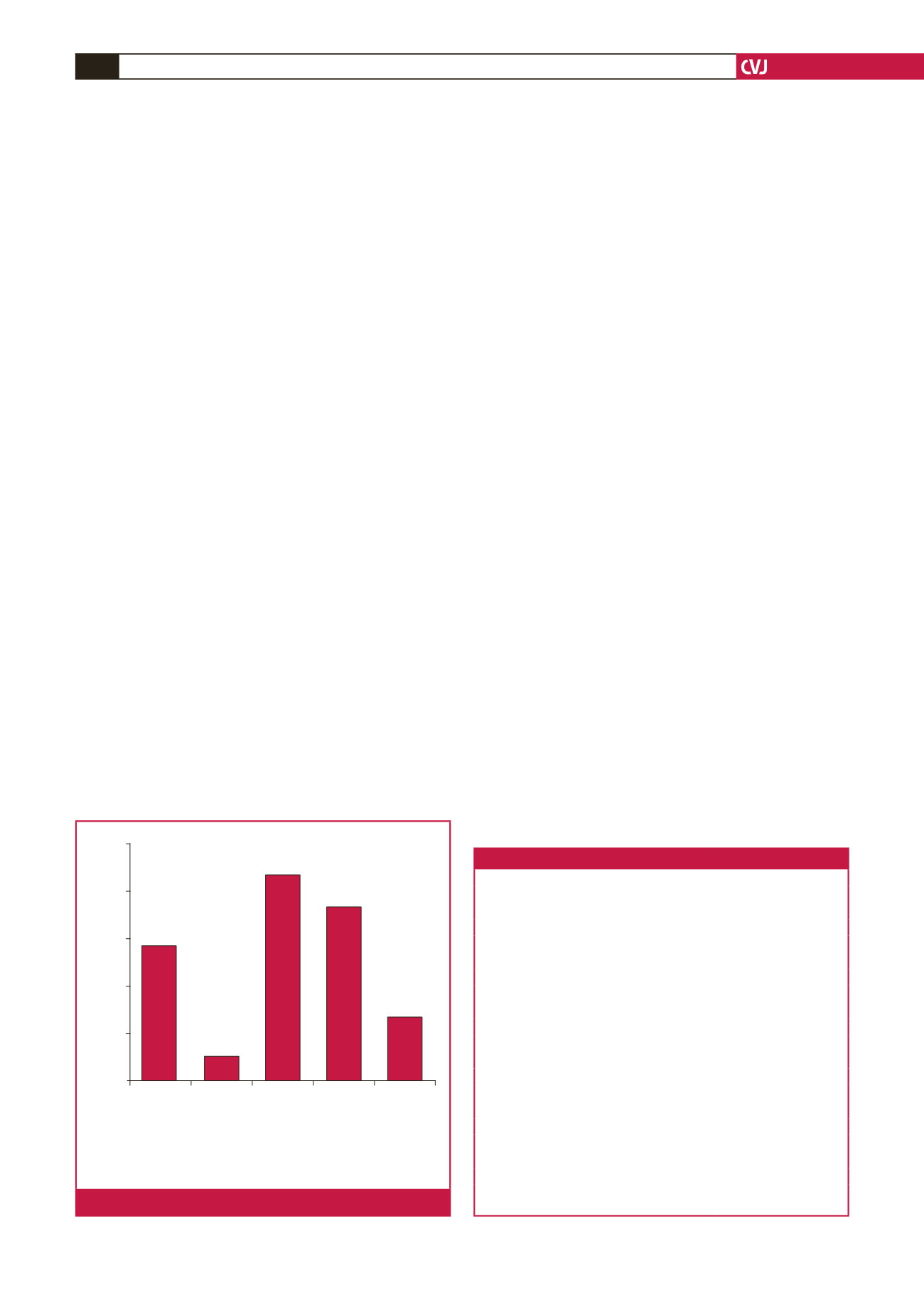
dextroversion. The extra-cardiac causes of dextroposition are
described in Fig. 1.
The median age at diagnosis of a RSH was two months
(range, prenatal to 16 years). The majority of diagnoses were
made before one year of age (144 out of 186 patients, 77.4%).
There were 97 male patients and 83 female patients. The
gender of six neonates was not documented. The male-to-female
ratio (180 patients) was 1:0.86 (53.9% male, 46.1% female).
ACM
Eighty-eight out of the 108 patients (81.5%) with dextrocardia
demonstrated ACM. The various ACM are listed in Table 1.
Of the 76 patients with dextroposition, four demonstrated
ACM (5.3%). An atrial septal defect secundum was diagnosed
in one patient and a large patent ductus arteriosus (PDA) in
another. A further two patients in this subgroup demonstrated
a small PDA in association with a hypoplastic right lung and
Scimitar syndrome. There were 11 patients with dextroposition
secondary to congenital diaphragmatic hernias (14.5%), none
of whom had documented ACM. One out of the two patients
in this subgroup had transposition of the great arteries (TGA).
Situs
Fifty-two patients with dextrocardia (Fig. 2) exhibited situs
inversus (48.1%) and ACM was diagnosed in 32 of them
(61.5%). Situs solitus was found in a further 24 patients
(22.2%), 15 of whom demonstrated ACM (62.5%). There was
A space-occuping lesion was defined as a mass
or tumour that causes local pressure leading to
displacement of the heart to the right hemi-thorax
Collapsed
right lung
Hypoplastic
lung
Space-
occupying
lesion
Scimitar
syndrome
Unrecorded
Number of patients
30
24
18
12
6
0
17
3
26
22
8
Fig. 1.
Extra-cardiac causes of dextroposition.
Table 1. Dextrocardia: associated cardiac malformations
15
Associated cardiac malformations
Number of patients (%)
Heterotaxias
18 (16.7)
Single ventricle
17 (15.7)
Hypoplastic left ventricle
2 (1.9)
Tricuspid atresia
6 (5.6)
Double-outlet right ventricle
13 (12)
D-transposition of great arteries
15 (13.9)
L-transposition of great arteries
2 (1.9)
Endocardial cushion defect
10 (9.3)
Total anomalous pulmonary venous return
4 (3.7)
Tetralogy of Fallot
3 (2.8)
Coarctation of the aorta
2 (1.9)
Ventricular septal defect
26 (24.1)
Pulmonary stenosis
19 (17.6)
Atrial septal defect secundum
10 (9.3)
Patent ductus arteriosus
11 (10.2)
No significant heart disease
36 (33.3)
Lung disease
8 (7.4)
Other (all other diagnoses)
8 (7.4)
Most patients demonstrated more than one ACM at the time of echocardio-
graphy

















