
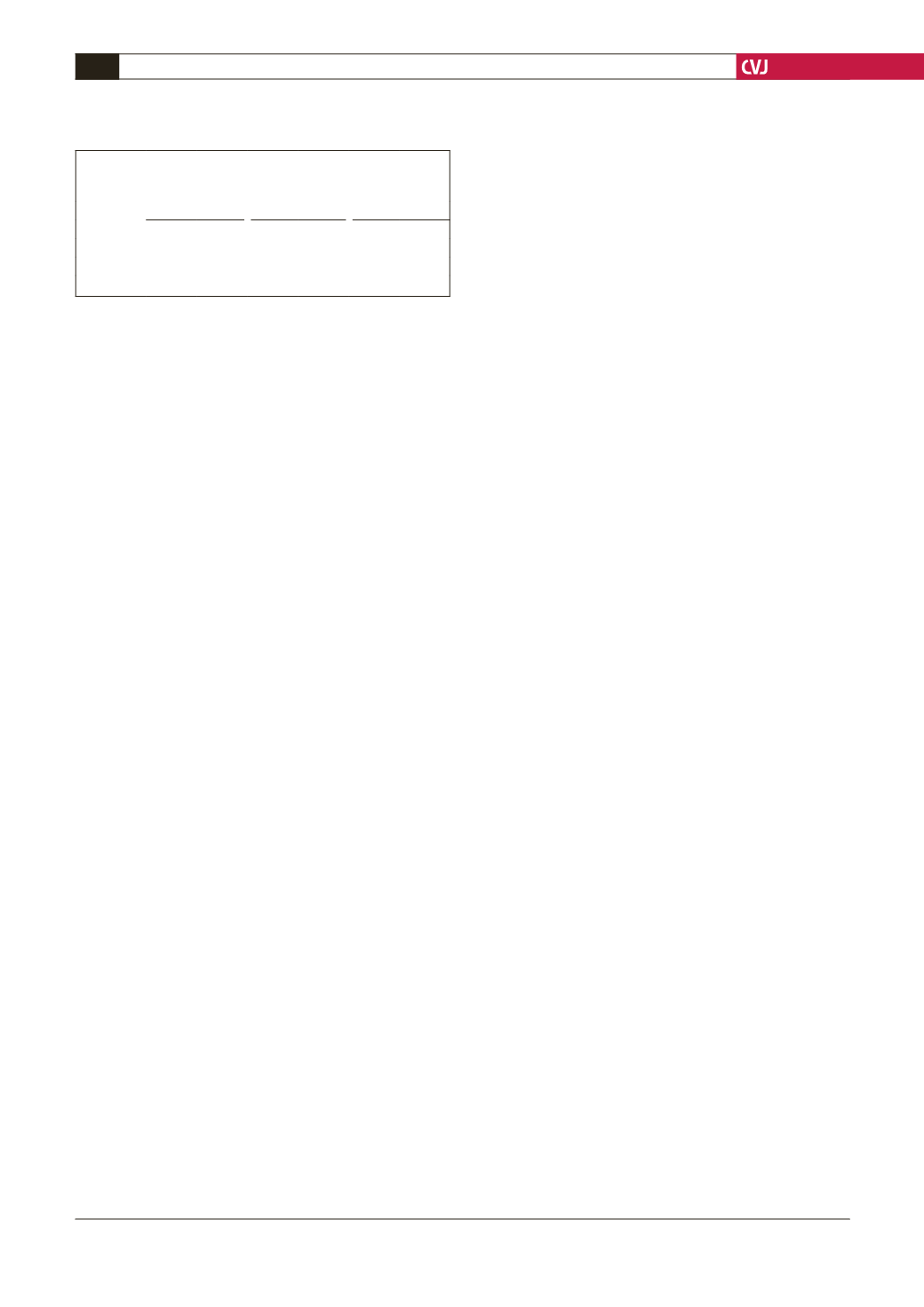
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 2, March 2013
18
AFRICA
Discussion
The prevalence of AAA in the general population aged 50 to
64 years seemed lower in Seychelles than in North America
or Europe. In North America, in participants aged 50–54/55–
59/60–64 years, the prevalence of AAA was 0.9/2.5/4.2% in
smokers and 0.2/0.5/0.9% in non-smokers, respectively.
4,7
In
Norway, the prevalence of AAA in men/women was 1.9/0% and
6.0/1.1% at ages 45–54 and 55–64 years, respectively.
13
In the
Netherlands the prevalence of AAA in men/women was 0.9/0.2%
and 3.1/0.4% at ages 55–59 and 60–64 years, respectively.
16
In contrast to what was recently described in a population of
mainly symptomatic aortic aneurysm patients in Kenya, we did
not find a female predominance for the diagnosis of AAA in
Seychelles. This was despite the predominant African descent of
the population and the prevalence of high blood pressure in the
50- to 64-year age category, which was the leading risk factor
associated with aortic aneurysms in this study.
17
This apparent
inconsistency might be due to methodological factors, such as
gender differences in health-related habits, since the Kenyan study
was based on hospital records and not on population-based data.
A low prevalence of AAA in Seychelles might be consistent
with a high prevalence of diabetes and the predominantly African
descent of the population, which are two factors reported to be
inversely associated with AAA.
6,7
It is however at odds with a
high prevalence of smoking (in men), high blood pressure and
hypercholesterolaemia in the Seychelles population.
Alternatively, we cannot exclude some imprecision in our
estimates in view of the relatively small size of our sample and
broad confidence intervals, although the population-based design
of the study as well as the high participation rate strengthens the
reliability of our epidemiological data. On the other hand, the
seemingly higher prevalence of aortic ectatic dilatation could
announce increasing rates of AAA in the next decades as the
population becomes exposed to high risk-factor levels over long
periods of time.
Furthermore, because of a high prevalence of AAA risk
factors, such as current smoking (28% in men and 4% in women
aged 40–49 years) or high blood pressure (35% in the 40–49-
year population) in younger age groups with a lower prevalence
of ‘protective factors’ such as diabetes mellitus (11.7% in
the 40–49-year population), ectatic lesions might appear at a
younger age in Seychelles than in North America or Europe.
However, given the small rate of expansion of small lesions over
time, the finding of true AAA in subjects aged less than 50 years
is unlikely.
18
Conclusion
Pending further data on the prevalence of AAA in older age
categories, our results do not support routine screening ofAAA in
the selected population. This is consistent with recommendations
for populations in Western countries.
5
References
1.
Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA,
et al
. Multicenter Aneurysm Screening Study Group. The Multicenter
Aneurysm Screening Study (MASS) into the effect of abdominal aortic
aneurysm screening on mortality in men: a randomized controlled trial.
Lancet
2002;
360
: 1531–1539.
2.
Norman PE, Jamrozik K, Lawrence-Brown MM, Le MTQ, Spencer
CA, Tuohy RJ,
et al
. Population based randomised controlled trial on
impact of screening on mortality from abdominal aortic aneurysm.
Br
Med J
2004;
329
: 1259–1264.
3.
Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdomi-
nal aortic aneurysms: single center randomised controlled trial.
Br Med
J
2005;
330
: 750–754.
4.
Fleming C, Witlock EP, Beil TL, Lederle FA. Screening for abdomi-
nal aortic aneurysm: a best evidence systematic review for the U.S
Preventive services Task Force.
Ann Intern Med
2005;
142
: 203–211.
5.
Lederle FA, Johnson GR, Wilson SE, Chute EP, Litooy FN, Bandyk
D,
et al
. Prevalence and associations of abdominal aortic aneurysm
detected through screening.
Ann Intern Med
1997;
126
(6): 441–449.
6.
Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS,
et al.
and the Aneurysm Detection and Management Veterans Affairs
Cooperative Study Investigators. The aneurysm detection and manage-
ment study screening program. Validation cohort and final results.
Arch
Intern Med
2000;
160
: 1425–1430.
7.
US Preventive Services Task Force. Screening for abdominal aortic
aneurysm: recommendation statement.
Ann Intern Med
2005;
142
:
198–202.
8.
Puech-Leao P, Molnar LJ, de Oliveira IR, Cerri GG. Prevalence of
abdominal aortic aneurysms – a screening program in Sao Paulo,
Brazil.
Sao Paulo Med J
2004;
122
(4): 158–160.
9.
Yii MK. Epidemiology of abdominal aortic aneurysm in anAsian popu-
lation.
ANZ J Surg
2003;
73
(6): 393–395.
10. Bovet P, Shamlaye C, Kitua A, Riesen WF, Paccaud F, Darioli R. High
prevalence of cardiovascular risk factors in the Seychelles (Indian
Ocean).
Arterioscler Thromb
1991;
11
(6): 1730–1736.
11. Bovet P, Romain S, Shamlaye C, Mendis S, Darioli R, Riesen W,
et al
.
Divergent 15-year trends in traditional and metabolic risk factors of
cardiovascular diseases in the Seychelles.
Cardiovasc Diabetol
2009;
8
: 34.
12. Bovet P, Shamlaye C, Gabriel A, Riesen W, Paccaud F. Prevalence of
cardiovascular risk factors in a middle-income country and estimated
cost of a treatment strategy.
BMC Publ Hlth
2006;
6
(1): 9.
13. Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of
and risk factors for abdominal aortic aneurysms in a population-based
study. The Tromso Study.
Am J Epidemiol
2001;
154
: 236–244.
14. Hirsch A, Haskal Z, Hertzer N, Bakal C, Creager M, Halperin J,
et al
.
ACC/AHA 2005 Practice guidelines for the management of patients
with peripheral arterial disease (lower extremity, renal, mesenteric, and
abdominal aortic).
Circulation
2006;
113
: e463–e654.
15. Basnyat PS, Aiono S, Warsi AA, Magee TR, Galland RB, Lewis MH.
Natural history of the ectatic aorta.
Cardiovasc Surg
2003;
11
(4):
273–276.
16. Pleumeekers HJCM, Hoes AW, van der Does E, van Urk H, Holman
H, de Jong PTVM, Grobbee DE. Aneurysm of the abdominal aorta
in older adults. The Rotterdam Study.
Am J Epidemiol
1995;
142
:
1291–1299.
17. Ogeng’o JA, Olabu BO, Kilonzi JP. Pattern of aortic aneurysms in an
african country.
J Thorac Cardiovasc Surg
2010;
140
: 797–780.
18. Brady A, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT for
the UK Small Aneurysm Trial Participants. Abdominal aortic aneurysm
expansion: risk factors and time intervals for surveillance.
Circulation
2004;
110
: 16–21.
TABLE 1. PREVALENCE OFANEURYSM OR ECTASY
OF THEABDOMINALAORTA IN THE GENERAL
POPULATION OF SEYCHELLESAGED 50–64YEARS
Men (
n
= 151)
Women (
n
= 178)
Total (
n
= 329)
% 95% CI
% 95% CI
% 95% CI
Aneurysm 0.7 0–2.0 0
0.3 0–0.9
Ectasy
2.0 0–4.2 0.6 0–1.7 1.2 0–2.4
Either
2.7 0.1–5.2 0.6 0–1.7 1.5 0.2–2.8



















