
AFRICA
S45
CVJAFRICA • Volume 26, No 2, H3Africa Supplement, March/April 2015
not yet fully explored. The H3Africa Consortium, with funding
support from the National Institutes of Health (NIH) and the
Wellcome Trust, is currently executing 24 different disease-based
projects involving 50 000 to 75 000 participants across the African
continent.
11
This initiative will deeply enhance our understanding
of human genomic variation while unravelling the genomic
bases of several communicable and non-communicable diseases
on the continent, while facilitating genomic infrastructural
development and capacity building.
The H3Africa Consortium is revolutionising genomic research
in Africa and closing the huge genomics gap between Africa and
the developed world. The initiative will reduce health disparities
and enhance understanding of health issues for the benefit of
Africans and the human race through the discovery of new
genes and disease pathways with therapeutic and preventative
potentials.
The Stroke Investigative Research and Education Network
(SIREN) project is one of the H3Africa-funded projects. The
SIREN investigators propose to explore genomic factors in
stroke in 6 000 native West Africans (3 000 case–control pairs)
in comparison with 1 000 African Americans (80% of whom
are of West African ancestral origin) and 12 000 Americans
of European ancestry in the REGARDS study (comparison
among three tracks).
116,117
The wide genomic variation of African
populations offers a unique opportunity to identify novel
genomic variants with causal relationships to stroke across
different ethnic groups.
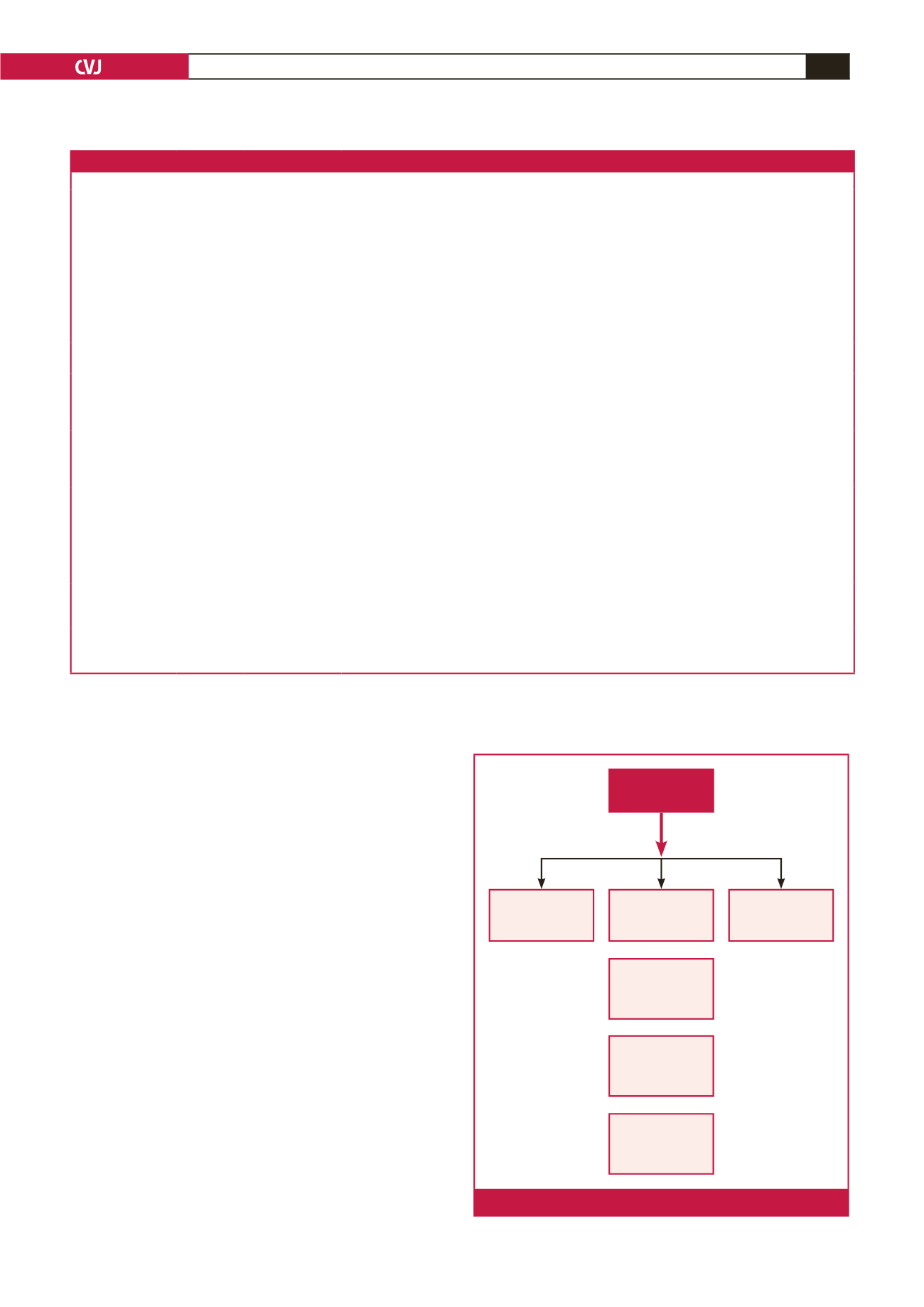
The SIREN project has three main streams: phenomics
(including community engagement), genomics, and
bio-informatics (Fig. 1). An ethnically diverse sample increases
the scope and generalisability of findings, because pan-ethnic
SIBS –
phenomics
SIBS –
genomics
SIBS –
Bio-informatics
Genomic
banking for
future analysis
Replication
phase
Discovery
phase
SIREN
Further analysis
with emerging
techniques
Accurate
phenotyping
of cases
Validate new
SNPs and CNVs
in REGARDS
black sub-cohort
WES
GWAS
Candidate gene
Pathway/Network
analysis
3 000 case-control pairs
Fig 1.
SIREN component projects.
Table 3. Genetic studies of stroke in Africa
First author (year) Study type Stroke phenotype Sample
Salient findings
Saidi
et al.
(2007)
97
Genotyping lschaemic stroke 135 cases,
118 controls
(Tunisian)
Altered plasminogen activator inhibitor 1 (PAI-1) and tissue-type plasminogen
activator (tPA) levels:
Significant
↑
in PAI-1 and marked
↓
in tPA levels correlated with 4G/5G, but not
with -844G/A, PAI-1 variants
4G/4G carriers had reduced risk of stroke compared with other genotypes
Saidi
et al.
(2007)
98
Genotyping lschaemic stroke 216 cases,
282 controls
(Tunisian)
ApoE
ε
3 lower (0.546 vs 0.736;
p
<
0.001) in stroke vs control
ApoE
ε
4 higher (0.370 vs 0.181;
p
<
0.001) in stroke vs control
Prevalence of Apo
ε
4-containing phenotypes higher in:
• ischaemic versus haemorrhagic (
p
<
0.001)
• small-vessel versus large-vessel stroke cases (
p
<
0.001)
• increased need for statin drugs (
p
=
0.040).
Mourad
et al.
(2008)
105
Genotyping Sickle cell
anaemia
20 SCA cases, 10
controls (Egyptian)
Presence or ACE D allele significantly predisposed to stroke in children with
sickle cell anaemia (SCA).
Saidi
et al.
(2008)
99
Genotyping lschaemic stroke 216 stroke patients,
318 controls
(Tunisian)
Human platelet alloantigen (HPA) – 1 a/b (
p
<
0.001) and HPA-5 a/b (
p
<
0.001)
alleles were associated with stroke-susceptible genotypes: 1a/b-2a/a-3a/b-4a/a-5a/b
protective genotypes: 1a/a-2a/a-3a/a-4a/a-5a/a; 1a/a-2a/a-3a/b-4a/a-5a/a; 1a/b
-2a/a-3a/a-4a/a-5a/a; 1a/b-2a/a-3a/b-4a/a-5a/a)
Saidi
et al.
(2008)
100
Genotyping lschaemic stroke 329 cases,
444 controls
Lower human platelet alloantigen, HPA-1a (
p
<
0.001) and higher HPA-1b (
p
<
0.001) allele frequencies were seen in cases than control subjects.
Homozygosity for HPA-1b (
p
<
0.001) alleles was more prevalent in stroke cases
than in controls.
Saidi
et al.
(2009)
101
Genotyping lschaemic stroke 228 cases,
323 controls
Frequency of APOE
ε
3 allele and Apo E3/E3 genotype lower (
p
<
0.001) in stroke
vs controls
Frequency of Apo
ε
4 allele and genotypes (E3/E4 and E4/E4) elevated (
p
<
0.001)
in stroke vs controls
Higher proportion of Apo
ε
4-carrying + ACE Del/Del positive cases seen in
young (
<
50 years) patients (
p
=
0.012) and associated with large-vessel stroke (
p
=
0.035).
Saidi
et al.
(2009)
102
Genotyping lschaemic stroke 329 cases,
444 controls
Angiotensinogen AGT 174T/235M/-6A, AGT 174T/235T/-6G. AGT 174T/235T/-
6A and AGT 174M/235T/-6A haplotypes were significantly associated with an
increased risk of stroke.
Saidi
et al.
(2010)
103
329 IS patients,
444 controls
Endothelial nitric oxide synthase (eNOS) gene polymorphisms (298Asp allele and
298Asp/4b/-786T and 298Asp/4b/-786C haplotypes, and in addition identified
298Asp/4a/-786T haplotypes) were significantly associated with ischaemic stroke.



















