
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 6, November/December 2019
370
AFRICA
Results
The total number of respondents was 30 (18% of the people
contacted), representing 14 countries (Fig. 1). Most respondents
(87%) were doctors working in public referral centres. RHD was
the commonest indication for BPG administration (86%); other
reported clinical indications include syphilis and sickle cell disease.
BPG was reported to be not regularly available
by 30% of
respondents (Fig. 2). All but one respondent indicated BPG is
on the national essential-drug list (96.6%) and on the free-drug
list (58%). Oral penicillin is included on the essential-drug list in
65% and on the free-drug list in 40% of respondents’ countries.
Most respondents recognised that one to three brands are
available, but some countries reported 10 brands (Uganda), six
brands (Tanzania) and five brands (Mozambique). Reported
retail purchase price for a 1.2-million international unit (IU) vial
ranged between US$0.5 and US$1. In 10 countries (71%) BPG
was listed as a ‘free drug’.
Skin testing before BPG administration is practiced by 40%
of respondents’ centres. Skin testing is performed prior to the
first injection by 20% and before each injection by 20% of
respondents (Fig. 3). Skin testing is mostly done with dilute
BPG (85%). Only 30% use controls for skin testing. Positive tests
were observed by 20% of respondents. Centres that perform
skin testing were in Angola, Nigeria, Sudan, Egypt, Zambia and
Mozambique.
Of the respondents, 30% did not have a guide to the
administration of BPG in their centre. In centres with a guide,
utilisation of the resource was estimated at 80%.
Only 30% had emergency kits containing adrenaline available
when BPG is administered.
There was a large variation between countries in interval of
BPG injections for secondary prophylaxis. BPG was mostly given
four weekly (60%), but 10% of respondents were administering
BPG every two weeks.
Minor reactions were observed by 33% of respondents and
major reactions by 30%. Major reactions included death in six
cases reported from Nigeria, Zimbabwe, Rwanda, Sudan and
Tanzania.
With regard to health workers’ concerns and needs, 43%
of respondents reported that health workers do have concerns
about BPG administration. These concerns include worry about
reactions, pain, viscosity of the solution and the difficulty to
inject it. Twenty-three per cent of respondents reported that they
had concerns about the quality of BPG.
Half of respondents reported that they do not feel confident
to manage a patient with BPG allergy. Most respondents (86%)
would like to have a refresher course on BPG administration and
95% would like to have an administration guide.
Fig. 1.
Geographic location of respondents to the penicil-
lin survey in alphabetical order: 1. Angola; 2. Egypt;
3. Ethiopia; 4. Liberia; 5. Mozambique; 6. Niger; 7.
Nigeria; 8. Rwanda; 9. South Africa; 10. Sudan; 11.
Tanzania; 12. Uganda; 13. Zambia; 14. Zimbabwe.
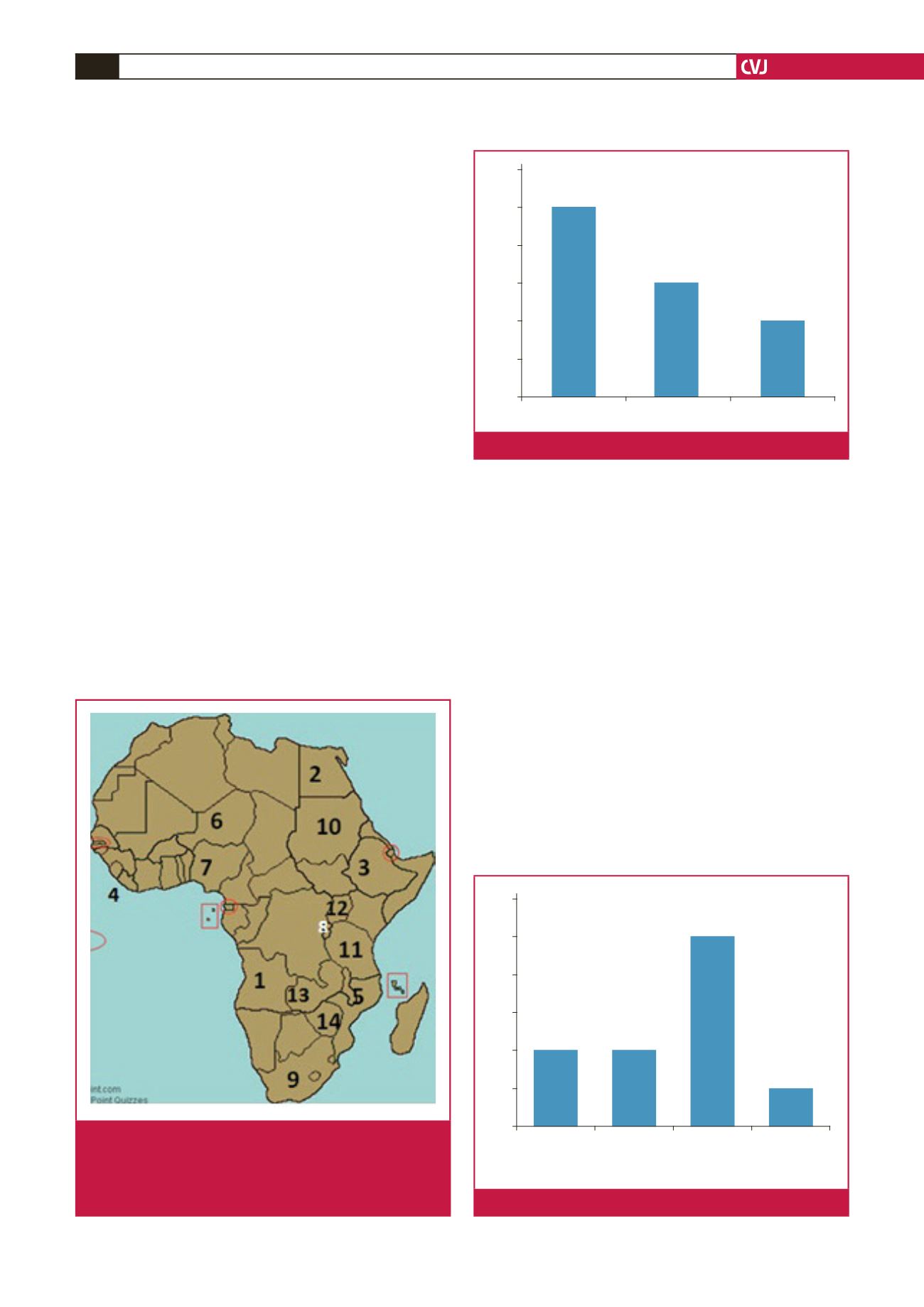
60%
50%
40%
30%
20%
10%
0%
BPG is available BPG is not available Don’t know
50%
30%
20%
Fig. 2.
Availability of benzathine penicillin (BPG).
60
50
40
30
20
10
0
Skin test
before each
injection
Skin test
before first
injection
Skin test not
done
No
information
Fig. 3.
Skin testing before BPG administration.



















