
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 25, No 5, September/October 2014
AFRICA
201
injection of 130 mg/kg of ketamine (Ketalar, Pfizer) and 20
mg/kg of xylasine (Rompun, Bayer). Sedation was maintained
with 50 mg/kg of ketamine hydrochloride so that the animals
remained under anaesthesia during blood collection and superior
mesenteric artery clamping.
Blood samples were obtained from the control group to
determine basal CNP levels. A simple laparotomy was performed
on the rats in groups I, II and III in order to clamp the superior
mesenteric artery (SMA) and artificially create mesenteric
ischaemia. The SMA remained clamped for three hours in group
I, six hours in group II, and nine hours in group III. Blood
samples were collected from the animals after the designated
duration of induced mesenteric ischaemia without declamping,
and then they were sacrificed. Several animals died during the
procedure, including one in group II and three in group III, and
they were subsequently excluded from the study. Plasma CNP
levels were measured from the collected blood samples.
Biochemical analysis was as follows. Blood collection tubes
containing citrate were used, and after the samples were obtained
they were centrifuged at 4 000 rpm at 4°C for 10 minutes. The
centrifuged samples were then transferred into Eppendorf tubes
for storage at –80°C.
Commercially available radioimmunoassay kits (RIA) (C-type
natriuretic peptide-22, Phoenix Pharmaceuticals, Belmount, CA,
USA) were used to determine plasma CNP levels. One millilitre
of plasma was eluted with a 1-ml volume of 60% acetonitrile
mixed in a 1% trifluoracetic acid (TFA) solution for the solid-
phase extraction step, as previously described by del Ray
et al.
7
After the remaining product was dissolved in 300–500
μ
l of
assay buffer, 100
μ
l of the resulting mixture was used to perform
the immunometric assay.
7
The average CNP recovery was
calculated to be 74.8%.
Statistical analysis
Statistical calculations were performed with the SPSS software
(SPSS version 15.0 for Windows, SPSS Inc., Chicago, IL USA).
Data were expressed as the mean
±
one standard deviation (SD).
The Kolmogorov–Smirnov test was used to assess whether the
data conformed to a normal distribution. A
p
-value
<
0.05
was considered statistically significant. Significant differences
between group means were assessed with one-way analysis of
variance (ANOVA). Tukey’s honest significant difference (HSD)
was used as a
post hoc
test.
Results
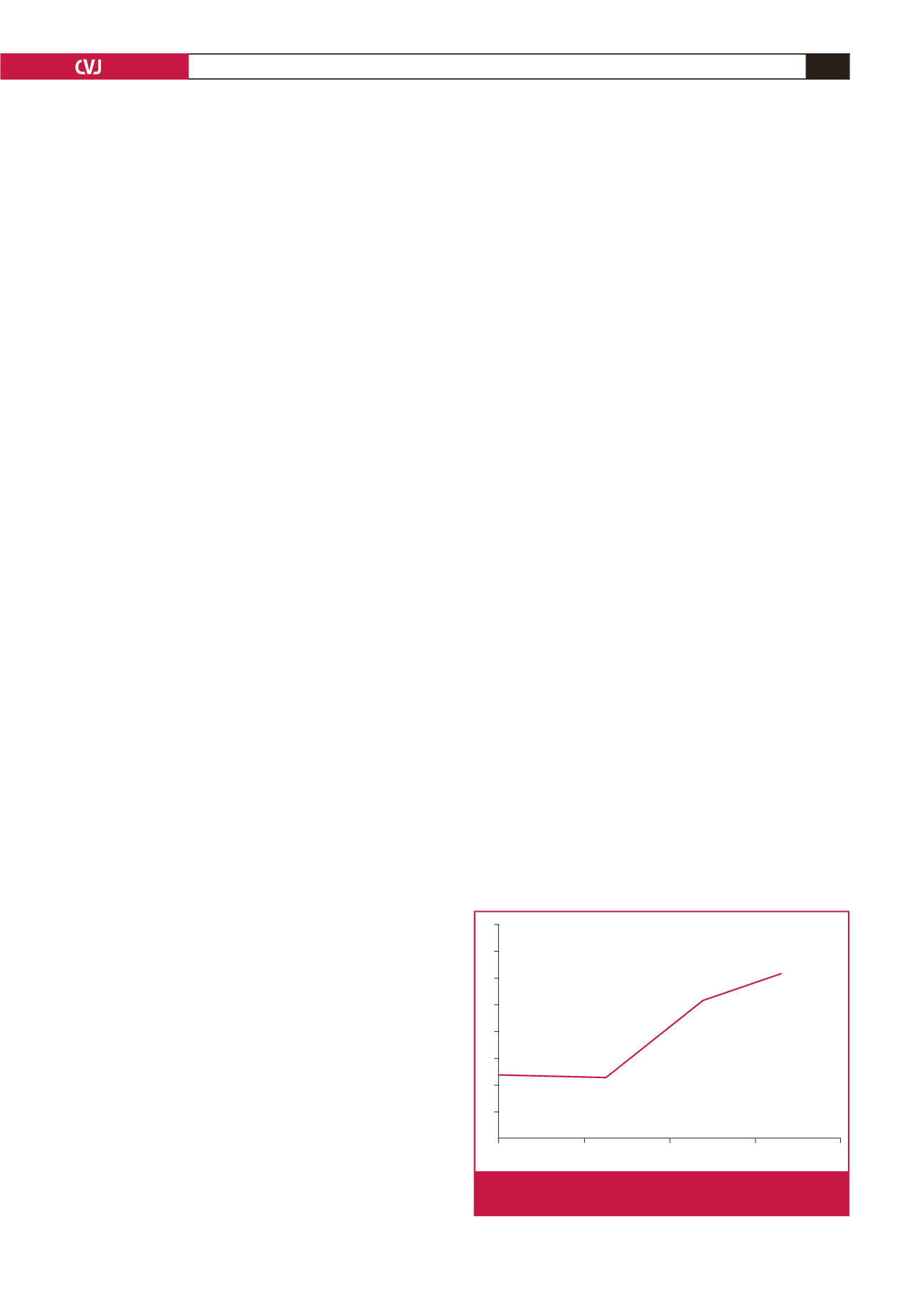
In the control group, the mean plasma CNP level was 2.54
±
0.42 pg/ml. A slight decrease in CNP level was observed in
group I relative to the controls following three hours of induced
mesenteric ischaemia [2.38
±
0.18 pg/ml (
p
=
0.085)]. However,
mean CNP levels were dramatically increased in group II (5.23
±
0.22 pg/ml) compared to the controls and group I following six
hours of mesenteric ischaemia (
p
=
0.001). Average CNP levels
were even higher in group III (6.19
±
0.67 pg/ml) relative to the
controls and group I (
p
=
0.000) and group II (
p
=
0.036).
There was a significant positive correlation between plasma
CNP levels and longer durations of induced mesenteric
ischaemia (
R
=
0.56,
p
<
0.001). The CNP levels observed in each
experimental group are summarised in Fig. 1.
Discussion
The findings of this study indicate that plasma CNP levels were
relatively low during the initial stages of mesenteric ischaemia.
However, CNP levels quickly elevated in response to longer
durations of sustained ischaemic injury. These findings are
promising because CNP levels may allow one to differentiate
between early and late mesenteric ischaemia.
The initial reduction in CNP levels during the early hours
of mesenteric ischaemia may have been due to systemic CNP
regulatory pathways. On the other hand, elevated plasma CNP
levels during the sixth and ninth hours of induced mesenteric
ischaemia may signify delayed mesenteric endothelial resistance
or a response compounded by progressively worsening mesenteric
ischaemia.
CNP was first isolated from blood collected from the brain
and was subsequently categorised into the natriuretic peptide
family, which contains three molecules that have a particular
22-amino acid structure.
9
In later studies, it was reported that
CNP may also be isolated from plasma samples obtained from
the colon, lung, heart and kidneys.
9
CNP is a unique endogenous ligand for natriuretic peptide
B receptor (NPR-B) and is upregulated by transforming growth
factor-
β
, which is an important vascular remodelling factor.
9,10
NPR-B is located on vascular smooth muscle and modulates
vascular tone.
9,11
CNP inhibited proliferation of endothelial and vascular
smooth muscle cells in
in vitro
studies.
12
Additionally, CNP
demonstrated anti-atherogenic properties via p-selection
suppression, which regulates the recruitment of leukocytes and
platelet–leukocyte transmission.
12
It has been reported that CNP is released from endothelial
cells in rat mesenteric vessels and activates endothelium-derived
hyperpolarising factor (EDHF). EDHF then triggers potassium
channel opening and NPR-B activation so that mesenteric
vascular smooth muscle cells will hyperpolarise and relax.
13
Despite the important role that CNP plays in mesenteric vessel
tone, the effects of CNP have not been previously studied in the
setting of mesenteric ischaemia.
CNP produced anti-fibrotic and anti-proliferative effects via
inhibition of cultured fibroblasts, and reduced tissue growth
8
7
6
5
4
3
2
1
0
Control
Group I
Group II
Group III
6.19
±
0.67 pg/ml
5.23
±
0.22 pg/ml
2.54
±
0.42 pg/ml
2.38
±
0.18 pg/ml
Fig. 1.
CNP levels according to duration of induced mesen-
teric ischaemia.



















