
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 6, November/December 2017
e2
AFRICA
A two- and three-dimensional transoesophageal echocardio-
gram (2D/3D TEE) was done to further define this lesion. In this
patient, poor transoesophageal views prevented a full assessment
of the pathology. There was a likely SA aneurysm with orifice
located below the non-coronary and left coronary cusps (NCC/
LCC). The aortic leaflets and the root were normal. There was
retraction of the aortic leaflets and impingement of the LA,
the right pulmonary artery, and possibly the right ventricular
outflow tract (RVOT) by the aneurysm. There were no associated
thrombi, vegetations or other congenital lesions.
Due to suboptimal imaging on TTE and TEE, a cardiac MRI
was requested to confirm the SA aneurysm and to define with
certainty its relationship to the surrounding structures (Fig. 2).
A 30 × 14-mm aneurysm with a 12-mm neck was noted below
the aortic valve, which extended to the LA roof (Fig. 3). The
communication point was just below the LCC. AR of moderate
severity was noted (Fig. 4).
Discussion
SA aneurysms are rare and postulated to be the result of a
defect of congenital origin between the valvular annuli and
the ventricular wall.
1
Other possible aetiologies, although not
confirmed, include tuberculosis, syphilis, rheumatic fever and
infective endocarditis.
3,4
Whether HIV has a causal connection
also remains to be proven. These infections may merely represent
an association, given their high prevalence, and causality cannot
be inferred.
5
SA aneurysms are rarer than sub-mitral aneurysms and the
diagnosis is more challenging.
1,2,6
They are mostly not suspected
clinically and are found coincidentally on imaging.
7
The chest
X-ray was normal in this patient, unlike with sub-mitral
aneurysms where an enlarged cardiac silhouette is often noted.
3
SA aneurysms need to be differentiated from more commonly
occurring aneurysms such as sinus of Valsalva aneurysms, which
are located above the aortic valve.
6
SA aneurysms mostly occur in young Africans.
6
The origin
is usually below the left coronary cusp, as in our patient.
2,6
Clinical presentation varies, ranging from cardiac failure and
systemic emboli (due to aneurysmal thrombi) to angina (due to
coronary artery compression or emboli), and dysrhythmias such
as ventricular tachycardia.
1
A lack of aortic cusp support and
distortion of the annulus is responsible for AR and subsequent
cardiac failure.
3
The most widely available imaging tool is TTE but this may
be inadequate, as smaller aneurysms may be missed, especially in
patients with suboptimal imaging windows. The diagnosis is also
dependent on the skill and knowledge of the operator.
7
Cardiac MRI is increasingly becoming a complimentary
tool to echocardiography in assessing valves and congenital
lesions.
8
In patients with inadequate echocardiographic imaging,
cardiac MRI allows a detailed assessment of the anatomy of
congenital lesions such as SA aneurysms, and their relationship
to the surrounding structures. Additionally, MRI allows accurate
quantification of AR severity.
Cardiac MRI in our case allowed the precise localisation of
the lesion below the LCC, which proved difficult on TTE and
2D TEE. Furthermore, involvement of the RVOT, which was
Fig. 3.
Left ventricular outflow tract (left) and short-axis views
(right) depicting compression of the left atrium (blue
arrow) by the sub-aortic aneurysm (red arrow).
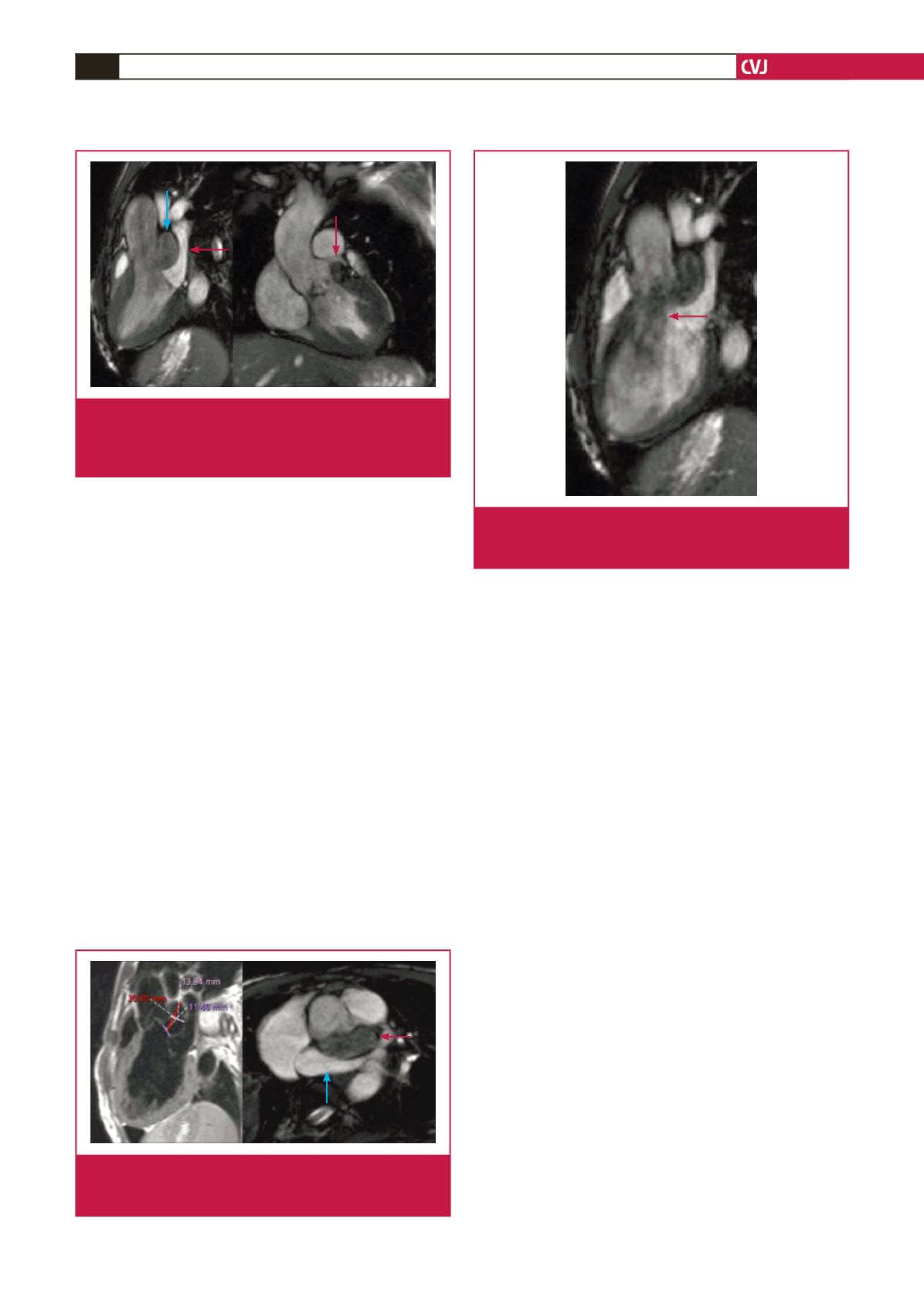
Fig. 2.
Left ventricular outflow tract views showing the sub-
aortic aneurysm (blue arrow) compressing the left
atrium (red arrow, left) and sparing the left coronary
artery (right).
Fig. 4.
Left ventricular outflow tract MRI image showing an
aortic regurgitant jet (arrow) secondary to retraction
of the left coronary cusp by the sub-aortic aneurysm.

















