
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 6, November/December 2018
354
AFRICA
IUCPQ directors, and patients were informed at the beginning
of the treatment that their evolution under this new medication
would be screened for evaluation for the medical quality act.
An informed consent was obtained from each patient and the
study protocol conformed to the ethical guidelines of the 1975
Declaration of Helsinki.
The objective of this study was to evaluate the usefulness of a
titration algorithm in patients naïve to sacubitril/valsartan. The
benefits of sacubitril/valsartan were clearly demonstrated in the
PARADIGM-HF study, but safety and ease of use still had to
be demonstrated in real-life practice.
The primary outcome was the proportion of patients able to
tolerate the maximal dose of 97/103 mg (200 mg) twice daily.
The secondary outcomes included the time needed to get to the
final titration, which corresponds to the maximal tolerated dose
for each patient, the variation of blood pressure from baseline
values, the incidence of symptomatic hypotension (defined as
any lowering in systolic blood pressure with related symptoms),
the incidence of hyperkalaemia (serum potassium increase to
>
5.5 mmol/l), the incidence of acute renal failure (30% increase in
creatinine level from baseline or more), variation of the baseline
treatment (beta-blocker, MRA and furosemide) daily dosage
from baseline to the final titration, and the need for down-
titration or discontinuation of sacubitril/valsartan.
Statistical analysis
Data are expressed using mean
±
standard deviation for
continuous variables or as a percentage for categorical data.
First and last measurements were analysed using a mixed model
as appropriate; the normality assumption was verified with the
Shapiro–Wilks tests on the error distribution from the Cholesky
factorisation. The results were considered significant with
p
-values
≤
0.05. All analyses were conducted using the statistical
package SAS v 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
From December 2015 to August 2016, 100 patients in whom
sacubitril/valsartan was initiated were titrated to the maximal
tolerated dose. Table 1 shows demographic and clinical baseline
characteristics of the patients. The majority of patients were
men with NYHA functional class II who had HF of ischaemic
aetiology. They were all on optimal tolerated medical therapy
and the mean systolic blood pressure was
±
16 mmHg. Twenty-
seven per cent of the patients were
≥
70 years old and 5% were
≥
80 years old.
Systolic blood pressure at baseline was
≤
110 mmHg in 25%
of patients and 35% had LVEF
≤
20%. Baseline creatinine was
≥
130 μmol/l in 21% of the cohort. Baseline ACEI/ARB dose was
≤
50% of the target dose recommended by practice guidelines in
41% of the patients.
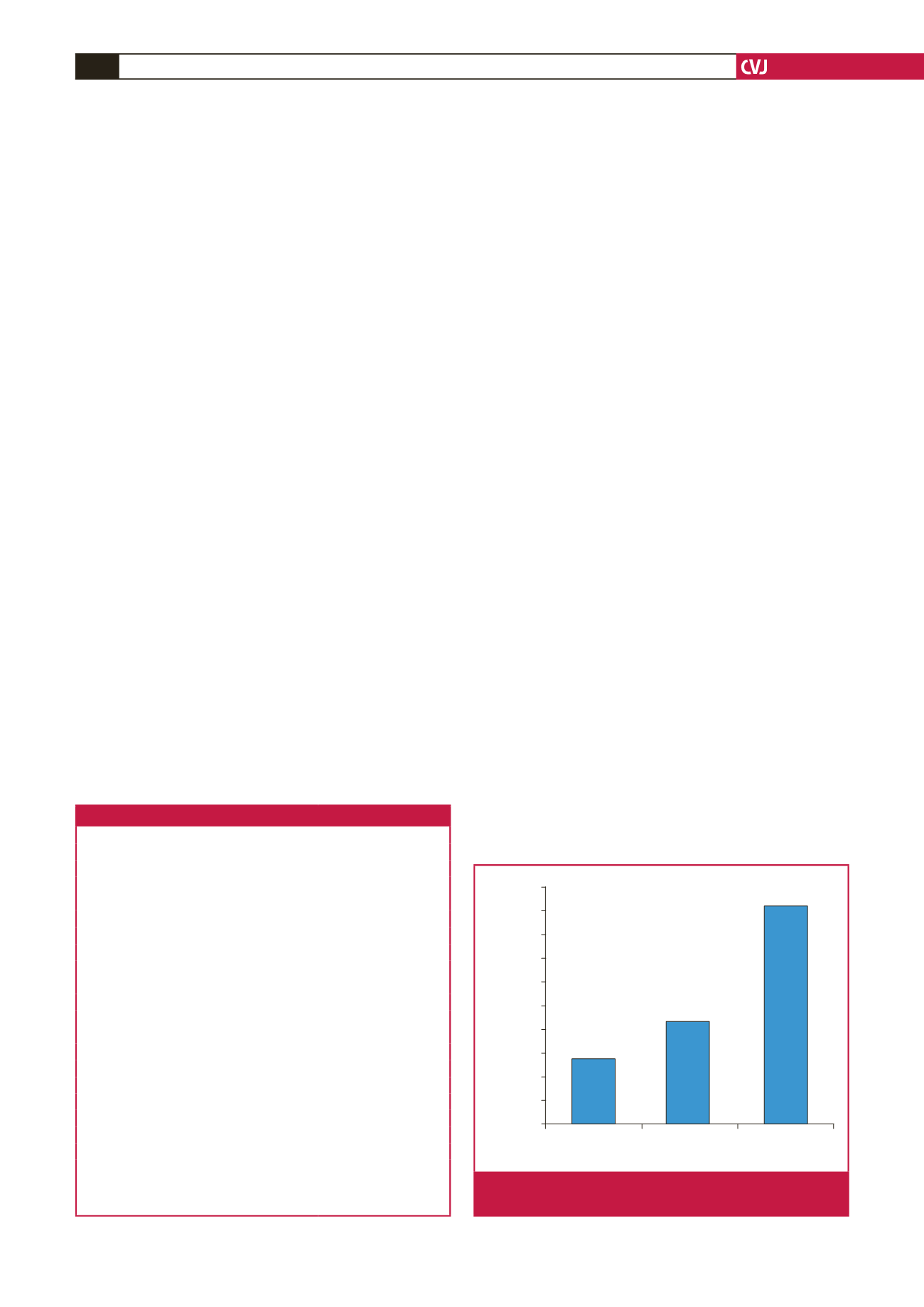
The sacubitril/valsartan maximal dose of 97/103 mg (200 mg)
twice daily was reached in 46% of patients and 73% had a dose
≥
49/51 mg (100 mg) twice daily at the final titration. The mean
final daily dose was 285
±
125 mg for a median value of 350 mg.
Sacubitril/valsartan dosage at the end of titration is reported in
Fig. 2.
Among the treated patients who tolerated up-titration (88
patients), 42 (47%) did not attain the maximal dose of 97/103 mg
twice a day. The most frequent reason for submaximal titration
was low blood pressure (25 patients). Titration was also limited
by orthostatic hypotension (three patients), dizziness (four
patients), fatigue (one patient), upper-limit potassium level (two
patients), ongoing diuretic titration (one patient), hospitalisation
for aortic and mitral valve replacement (one patient), lack of
adherence to the follow up (three patients), heart transplant (one
patient) and sulfasalazine-related hepatitis (one patient).
Mean titration time (drug initiation to maximal tolerated
dose) was 30
±
9 days. At the end of titration, mean systolic
blood pressure was 110
±
16 mmHg, which represents a
reduction of 12 mmHg (9.8%) when compared to the baseline
value (
p
<
0.0001).
Modification of baseline HF therapy during the on-treatment
phase was also recorded (Table 2). The mean daily dose of loop
Table 1. Baseline characteristics
Characteristic
Number
=
100
Mean age (years)
64
±
11
Male gender (%)
76
Ischaemic cardiomyopathy (%)
56
Mean LVEF (%)
26
±
7
Mean creatinine level (μmol/l)
104
±
29
Mean systolic blood pressure (mmHg)
122
±
16
Functional class
NYHA I (%)
1
NYHA II (%)
73
NYHA III (%)
26
NYHA IV (%)
0
ICD (%)
70
CRT (%)
40
Medical treatment
ACEI (%)
71
ARB (%)
29
Beta-blocker (%)
98
MRA (%)
71
Loop diuretic (%)
80
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor
blocker; CRT: cardiac resynchronisation therapy; ICD: implantable cardiovert-
er–defibrillator; LVEF: left ventricular ejection fraction; MRA: mineralocorti-
coid receptor antagonist; NYHA: New York Heart Association.
Patients (%)
100
200
400
Total daily dose (mg)
50
45
40
35
30
25
20
15
10
5
0
Fig. 2.
Sacubitril/valsartan maximal tolerated dose at end of
titration.

















