
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 32, No 1, January/February 2021
24
AFRICA
were isolated VSDs detected in 32 (31.4%) neonates, followed
by isolated ASDs in 22 (21.6%). The ductus arteriosus was still
patent in 1 643 (42.6%) of the neonates at enrolment. The highest
frequency of ductal patency was in neonates recruited on the
first day of life (1 220/1 643, 74.3%), and decreased markedly by
the second and third days of life to 12.3% (202/1 643) and 6.5%
(107/1 643), respectively. By the sixth day of life, only 11 neonates
still had ductal patency. However, only seven neonates had a
ductal diameter that exceeded 1.5 mm, therefore satisfying the
criteria for classification as CHD; a prevalence of four per 1 000.
Five (71.4%) of the seven neonates had isolated PDAs while the
remaining two (28.6%) co-existed with other CHD; one ASD
and one VSD (Table 4).
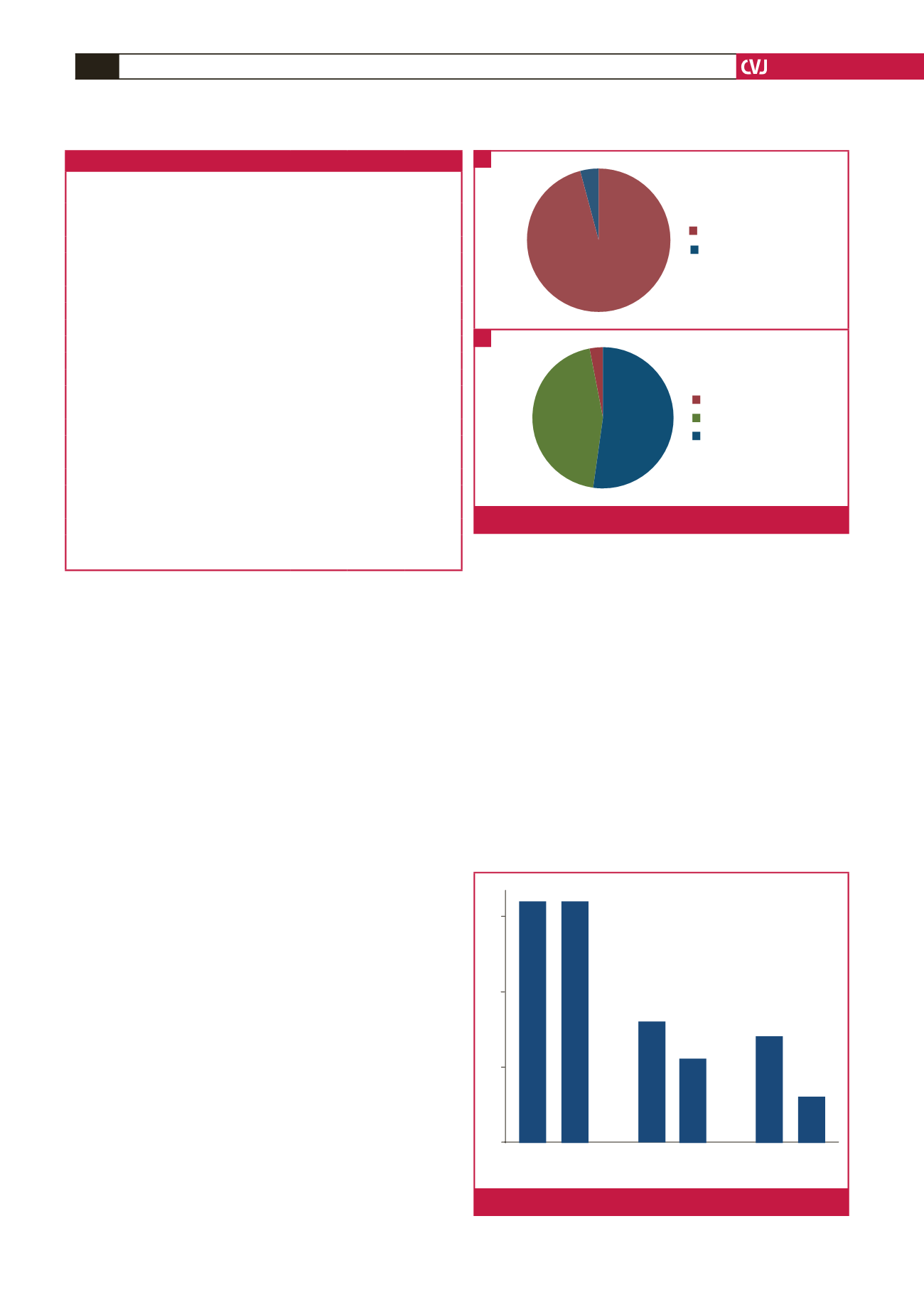
Isolated ASDs were present in 22 of the 24 (91.7%) neonates
with ASD; one ASD co-existed with a PDA and the other with
moderate pulmonary stenosis. Most (95.5%) of the isolated
ASDs were ostium secundum defects; one (4.5%) was an ostium
primum ASD. Thirty-two of the 34 (94.1%) neonates with VSD
had isolated lesions; the other two co-existed, one with a PDA
and the other with a mild pulmonary stenosis. The majority of
the isolated VSDs were peri-membranous defects, which were
detected in 17/32 (53.1%). Muscular VSDs were present in 14/32
(43.8%), while a sub-aortic VSD was detected in one (3.1%) of
the neonates with isolated VSD (Fig. 2).
Sixty four of the 111 neonates with CHD had mild defects
(16.6 per 1 000 population) while 27 had moderate CHD (7.0 per
1 000 population). Severe CHD was detected in 20 neonates with
a prevalence of 5.2 per 1 000. Moderate and severe CHD were
more common in males; 16/64 (25.8%) male versus female 11/49
(22.4%) and 14/64 (22.6%) male versus female 6/49 (12.3%),
respectively, but these were not significantly different (
p
= 0.75
and
p
= 0.08, respectively). More female neonates (32/49 or
65.3%) had mild CHD compared with males: 32/64 or 51.6%,
but the difference was also not significantly different;
p
= 0.11
(Fig. 3).
Mild CHD was present in 42 of the 2 340 neonates delivered
in the study hospitals (17.9 per 1 000 live births) while moderate
and severe lesions were present in 14 (6.0 per 1 000 live births)
and eight (3.4 per 1 000 live births) patients, respectively.
Admitted neonates with CHD had more severe lesions
compared with those who were not admitted (
p
= 0.001). There
was no significant association between the severity of CHD and
gender, place of delivery, maternal age, gestational age at delivery
or birth weight (Table 3).
Mothers of neonates with CHD were older (30.6 ± 5.6 vs 28.4
± 6.8 years,
p
< 0.001) and had higher parity (3.5 ± 2.0 vs 2.8 ±
1.9,
p
< 0.001). Neonates with CHD had lower birth weight (2.8
± 0.6 vs 3.1 ± 0.6 kg,
p
< 0.001), were four times more likely to
receive in-patient care [OR 4.4 (95% CI 2.8–6.8),
p
< 0.001] or
had dysmorphic features (
p
< 0.001). There was no difference
in mean gestational age between neonates born with CHD and
0
10
Male Female
Mild
Male Female
Moderate
Male Female
Severe
20
30
Fig. 3.
The distribution of CHD based on severity
Table 4. Prevalence and spectrum of CHD
Total
(3 857)
Male
(2 016)
Female
(1 841)
Spectrum of CHD
n
per 1 000
n
per 1 000
n
per 1 000
Acyanotic CHD
102 (26.4) 55 (27.3) 47 (25.5)
Atrial septal defect
24 (6.5)
10 (5.0)
14 (7.6)
Ventricular septal defect
34 (9.2)
16 (7.9)
18 (9.8)
Combined atrial and ventricular septal defect 13 (3.4)
10 (5.0)
3 (1.5)
Atrioventricular septal defect
5 (1.3)
3 (1.5)
2 (1.1)
Isolated pulmonary stenosis
9 (2.3)
7 (3.5)
2 (1.1)
Isolated patent ductus arteriosus
5 (1.3)
2 (1.0)
3 (1.5)
Coarctation of the aorta
4 (1.1)
2 (1.0)
2 (1.1)
Bicuspid aorta valve
4 (1.1)
3 (1.5)
1 (0.5)
Aortopulmonary window
1 (0.3)
1 (0.5)
0 (0.0)
Double-outlet right ventricle
1 (0.3)
0 (0.0)
1 (0.5)
Partial anomalous pulmonary venous return 1 (0.3)
1 (0.5)
0 (0.0)
Cortriatriatum dexterum
1 (0.3)
0 (0.0)
1 (0.5)
Cyanotic CHD (severe)
9 (2.3)
7 (3.5)
2 (1.1)
Tetralogy of Fallot
1 (0.3)
0 (0.0)
1 (0.5)
Truncus arteriosus
1 (0.3)
1 (0.5)
0 (0.0)
Ebstein’s anomaly
1 (0.3)
1 (0.5)
0 (0.0)
Critical CHD
Hypoplastic left heart syndrome
4 (1.1)
3 (1.5)
1 (0.5)
Total anomalous pulmonary venous return 1 (0.3)
1 (0.5)
0 (0.0)
Transposition of great arteries
1 (0.3)
1 (0.5)
0 (0.0)
Otium primum
Ostium secundum
Perimembranous
Sub-aortic
Muscular
Fig. 2.
A. Types of ASD detected. B. Types of VSD detected.
A
B



















