
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 32, No 1, January/February 2021
34
AFRICA
was too large for the largest available device, or the anatomy
was deemed unsuitable to safely release the device. In only three
cases was a device actually opened and not deployed, giving an
implant success rate of 97.6% (122/125).
Eight patients were lost to follow up between 30 days and three
years post procedure. The remaining 114 patients were followed
up to the end of March 2020 or had died, giving complete data
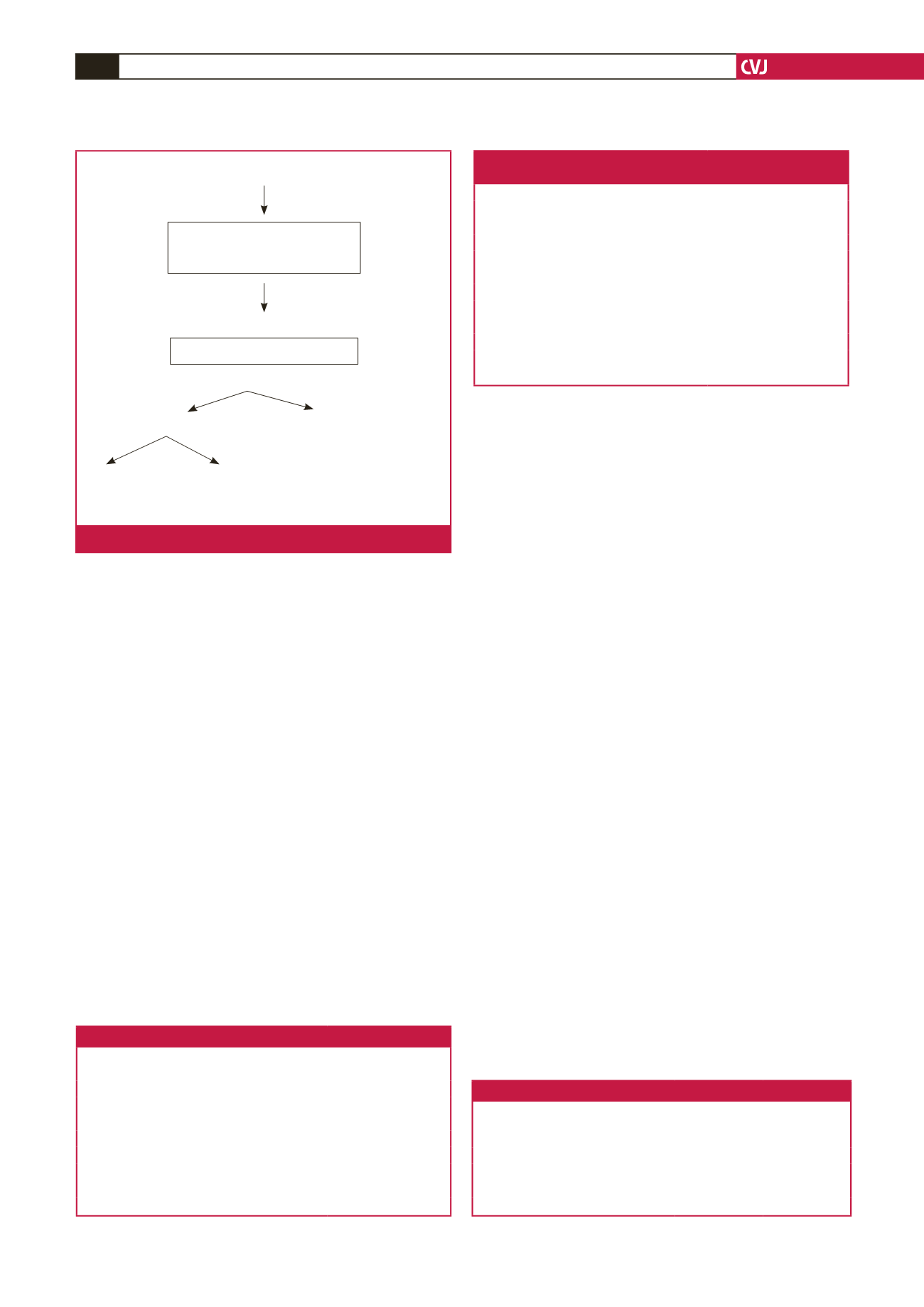
on 93.4% of patients who had had a device implanted (Fig. 1).
The average age of patients was 74.2 years (range 51–87; SD
8.1; median 75) and the average length of follow up was 38.5
months (range 1.2–111.5 months; SD 26.8; median 35.7); 75% were
male. The average CHADS
2
-VASc score was 3.9 (SD 1.2; median
4) and HAS-BLED score was 2.99 (SD 0.95; median 3) (Table 1).
In 71% (81) of patients, OACT was contra-indicated due to a
previous life-threatening bleed, while 9% (10) had a high bleeding
risk (HAS-BLED score
>
3). In 20% (23) of patients, LAAO was
indicated due to a combination of frailty not measured on
HAS-BLED, repeated falls or lifestyle choice. Previous stroke
or transient ischaemic attack (TIA) had occurred in 23% (26) of
patients (Table 1).
There was one major complication (
<
1%). An Amulet device
embolised to the descending aorta shortly after its release and
was successfully removed percutaneously via the right femoral
artery. However, the patient did sustain radiation burns due to
the complexity of retrieving the device. There were no deaths,
pericardial effusions requiring aspiration or strokes.
There were nine minor complications, including one
pericardial effusion seen at seven days, which was treated
conservatively as the patient was haemodynamically stable with
no evidence clinically or on TTE of cardiac tamponade. Seven
patients had a minor bleed from the femoral vein puncture
site that required a superficial skin suture to be placed post
procedure. One patient had a TIA during a difficult procedure
due to very awkward anatomy of the LAA. The procedure was
eventually abandoned as the anatomy was deemed unsuitable for
LAA closure (Table 2).
There were no significant leaks (
>
5 mm) past the device. If a
significant leak was seen on either TOE or left atrium angiogram
immediately after device deployment, the device was either
redeployed in a different position or the device size was changed.
There were 14 (11%) device size changes.
One hundred and twenty patients (96%) were discharged on
dual antiplatelet therapy (DAPT) for one month, and thereafter
reduced to low-dose aspirin only. Five patients were discharged
on aspirin only. The average length of hospital stay was 1.1 days
(one to six days).
A total of 35 (30.7%) patients died during the follow-up study
period (average of 2.5 years post procedure), ranging from 96 days
post procedure (primary amyloidosis not previously diagnosed)
to 2 700 days (seven years five months at the age of 86 years).
There were six strokes (5.3% of total or 1.7% per year). The
average CHADS-VASc score was 4, and four patients died due to
the stroke or consequences thereof. The majority of patients died
from cardiovascular causes (heart failure, myocardial infarction,
sudden cardiac death), cancer, renal failure and complications
arising from a fall. Three patients had a TIA.
There were 10 major bleeding events (8% or 2.8% per year).
Two occurred while on DAPT soon after the procedure, resulting
in the withdrawal of clopidogrel, while eight occurred while on
aspirin only, resulting in cessation of all antiplatelet therapy. In
Table 2. Adverse outcomes up to seven days post procedure
(
n
= 125 patients)
Adverse outcomes
Number (%)
Major
Death
0
Cardiac tamponade
0
Stroke
0
Device embolisation
1 (0.8)
Vascular injury
0
Bleed
0
Minor
Transient ischaemic attack
1 (0.8)
Bleed – groin puncture site
7 (5.6)
Pericardial effusion (not treated)
1 (0.8)
131 patients had general anaesthesia in cath lab for planned LAAO
6 patients withdrawn due to LAA
thrombus, LAA too large or inability
to pass TOE probe
125 patients, LAAO attempted
3 patients, procedure abandoned
122 patients enrolled in database (97.6% success)
114 complete follow up (93.4%)
8 lost to follow up
(30–1 095 days post procedure,
average 795 days. All were well
when last seen)
35 died
79 alive
(30.7%)
(69.3%)
Fig. 1.
Patients enrolled.
Table 1. Patient characteristics (
n
= 131)
Characteristics
Values
Age (years) (range, SD, mean)
74.2 (51–87, 8.1, 75)
Male:female (%)
75:25
CHADS
2
-VASc score (SD, median)
3.9 (1.2, 4)
HAS-BLED score (SD, median)
2.99 (0.95, 3)
Previous stroke/TIA (%)
23
Previous major bleed (%)
71
High bleeding risk (HAS-BLED
>
3) (%)
9
Frail, falls, lifestyle choice (%)
20
TIA: transient ischaemic attack.
Table 3. Long-term outcomes (
n
= 114 patients)
Long-term outcomes
Total number
Total %
(% per year)
Death
35
30.7
Stroke
6
5.3 (1.7)
Major bleed
10
8 (2.8)
Single antiplatelet agent at last follow up
94
82.5
No antiplatelet agent at last follow up
17
14.9



















