
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 32, No 1, January/February 2021
AFRICA
35
total, 17 patients (14.9%) were on no antiplatelet therapy at long-
term follow up. None of these patients had an embolic event. At
the last follow up, 97.4% of the patients were on single (82.5%)
or no antiplatelet therapy (14.9%) (Table 3).
Discussion
This single-centre registry of LAAO using almost exclusively the
Amulet device is in line with previously published registries of
LAAO but has a longer period of follow up than most registries
and trials.
2-8
The indications for LAAO, age of the patients, and CHADS
2
-
VASc and HAS-BLED scores are very similar to other registries.
Currently, almost all patients receiving LAAO have a relative
or absolute contra-indication for OACT, with very few patients
either refusing OACT or not having it because of a lifestyle choice
due to high-risk activities. The current 2016 ESC guidelines list
LAAO as a class 2b indication procedure.
1
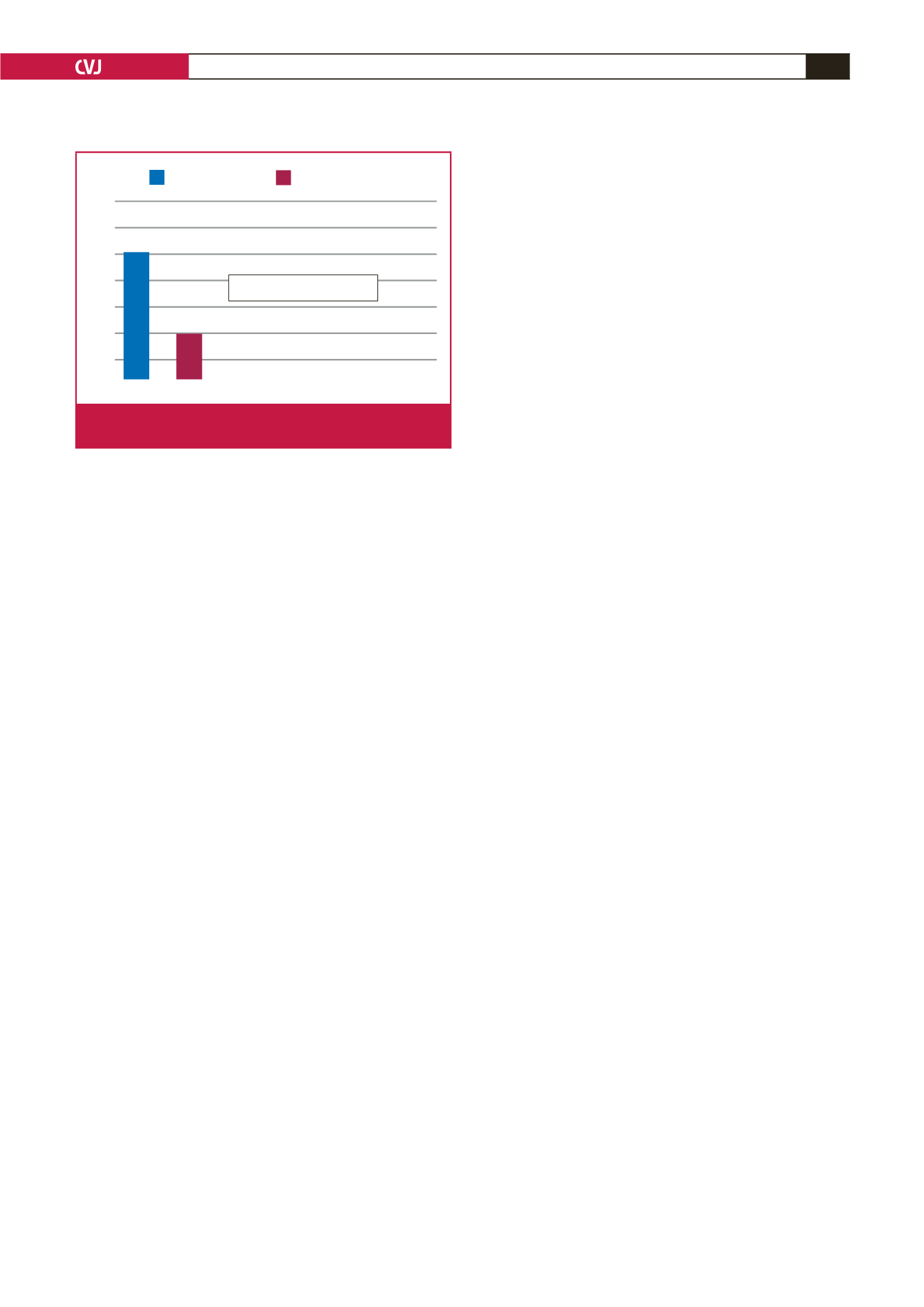
There were six strokes (stroke incidence 5.3%; 1.7% per
year) documented during follow up, with four fatalities (Fig. 2).
These occurred between six months and four years after LAAO
implantation (average 2.6 years). All these patients were on
low-dose aspirin only at the time of the stroke. The predicted
stroke rate per year according to a CHADS
2
-VASc score of 3.9
is 4.8%. This represents a 65% stroke risk reduction. This is in
keeping with other published registries and trials on LAAO
showing equivalence with warfarin
2-8
and the newer direct
OACT.
16,17
There were three TIAs and no other documented
thrombo-embolic (TE) events. (TE incidence was 7.9%; 2.5% per
year). The predicted TE risk was 6.7%, equating to a 63% risk
reduction.
There was no routine use of TOE at six to 12 weeks post
device implant as there is currently no clear evidence linking the
presence of device-related thrombus (DRT) and systemic embolic
events.
14,15
Furthermore, starting patients on OACT to manage
DRT carries significant risk in this particular group of patients
in whom OACT was contra-indicated in over 80% of patients.
Only two of the six stroke patients were managed at our
hospital. Both had a TOE post stroke. No DRT was seen in
either patient. One patient who presented with a small stroke at
three months post LAAO implantation was subsequently found
to have a severe ipsilateral internal carotid artery stenosis, which
was successfully stented. A year later, he suffered a further small
stroke and TOE showed the Amulet device had shifted slightly
and was partially protruding from the LAA orifice. Although no
DRT was seen, the patient was started on lifelong OACT and
remained well three years later.
A further patient who had a TIA a year post LAAO was
found to have a significant patent foramen ovale (PFO) on TOE.
There was no device-related thrombus, and the device was well
seated and fully endothelialised. The PFO was subsequently
closed percutaneously.
The overall mortality rate was 30.7%, however, this is not
unexpected for this population of patients with AF who were
elderly (average age 74 years; SD8.1), hadmultiple co-morbidities
(CHADS
2
-VASc 3.9), and were followed up for a prolonged
period of time (3.2 years; SD 2.17). The expected mortality rate
in patients with AF is two to four times higher than the average
population and worsens as the CHADS
2
-VASc score increases.
9-13
The majority of patients died from cardiovascular causes or
malignancy, which is in keeping with reported literature.
Limitations of this study include a single-centre, single-
operator registry with a limited number of patients enrolled.
Eight patients were lost to follow up and were not included in
this registry. Not all patients were followed up by the operator
and it is possible some embolic events were not reported.
Conclusion
This single-centre registry showing follow up over a prolonged
period of time confirms the efficacy of LAAO as an acceptable
alternative to OACT.
References
1.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B,
et al.
;
ESC scientific document group. 2016 ESC guidelines for the manage-
ment of atrial fibrillation developed in collaboration with EACTS.
Eur
Heart J
2016;
37
: 2893–2962.
2.
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M,
et al.
; PROTECT AF investigators. Percutaneous closure of the left atrial
appendage versus warfarin therapy for prevention of stroke in patients
with atrial fibrillation: a randomised non-inferiority trial.
Lancet
2009;
374
: 534–542.
3.
Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK,
et al
.
Prospective randomized evaluation of the Watchman left atrial append-
age closure device in patients with atrial fibrillation versus long-term
warfarin therapy: the PREVAIL trial.
J Am Coll Cardiol
2014;
64
: 1–12.
4.
Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K,
et al.
;
PREVAIL and PROTECT AF investigators. 5-year outcomes after left
atrial appendage closure: from the PREVAIL and PROTECT AF trials
.
J Am Coll Cardiol
. 2017;
70
: 2964–2975.
5.
Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B,
et
al.
; for the EWOLUTION investigators. Evaluating real-world clinical
outcomes in atrial fibrillation patients receiving the WATCHMAN left
atrial appendage closure technology: final 2-year outcome data focus-
ing on history of stroke and hemorrhage.
Circ Arrhythm Electrophysiol
2019;
12
(4).
6.
Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G,
et al.
Left atrial appendage occlusion for stroke prevention in atrial fibril-
lation: multicentre experience with the AMPLATZER Cardiac Plug.
EuroIntervention
2016;
11
: 1170–1179.
Predicted stroke rate
Actual stroke rate
6
5
4
3
2
1
0
4.8%
1.7%
65% Stoke rate reduction
Fig. 2.
Observed versus predicted stroke rate per year
(CHADS
2
-VASc score 3.9).



















