CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 2, March/April 2010
AFRICA
75
Javaux
et al
.
40
reported in 1995 that rabbit cardiomyocytes
were unable to phosphorylate AICAR to ZMP. Because of the
observed inability ofAICAR to elicit glucose uptake in rat cardio-
myocytes, we tested the ability of ZMP to affect glucose uptake.
As seen in Fig. 2A, ZMP also significantly lowered basal glucose
uptake levels 0.6
±
0.22-fold (
p
<
0.05). To ascertain that this was
not because AICAR or ZMP influenced cell viability, PI staining
was performed at the end of the experimental protocol. Fig. 2B
shows that cell viability was not affected by either substance.
GLUT4 translocation in cardiomyocytes
Because of the observed activation of AMPK by AICAR and
ZMP, but not of glucose uptake, we assessed GLUT4 transloca-
tion under these conditions, fractionating the cells into cytosolic
and membrane compartments, and then probing Western blots
of the separated proteins with a specific GLUT4 antibody. As
shown in Fig. 3A and B, a significant increase in GLUT4 could
be seen between basal and stimulated cells in the membrane
compartment (1.01
±
0.01 arbitrary densitometry units vs insulin
1.45
±
0.2, AICAR 1.29
±
0.10 and ZMP 1.56
±
0.1).
Determination of GLUT4 exofacial loop
In order to understand this discrepancy, we used an antibody
directed against the exofacial loop of the GLUT4 protein,
coupled to flow cytometry, to determine whether the protein was
properly inserted into the membrane.
41
As seen in Fig. 4, these
of AMPK, the ability of these compounds to elicit phosphoryla-
tion of the kinase was determined. This was accomplished by
measuring AMPK activation in terms of its phosphorylation on
Thr
172
via Western blotting and a specific antibody. In cardiomyo-
cytes treated with AICAR (1 mM for 30 min) and ZMP (1 mM
for 30 min) it was found that both substances resulted in signifi-
cant phosphorylation of AMPK (Fig. 1A). Cells made anoxic by
incubation in medium equilibrated with nitrogen were used as a
positive control. To ascertain that the phosphorylated kinase was
active, the phosphorylation of one of its downstream substrate
proteins, acetyl-Co-A carboxylase (ACC) was determined on the
same samples. Fig. 1B is a representative blot showing phospho-
rylation of AMPK and ACC after stimulation with both AICAR
and ZMP, while Fig. 1C is a representative Ponceau red-stained
membrane showing equal loading of protein.
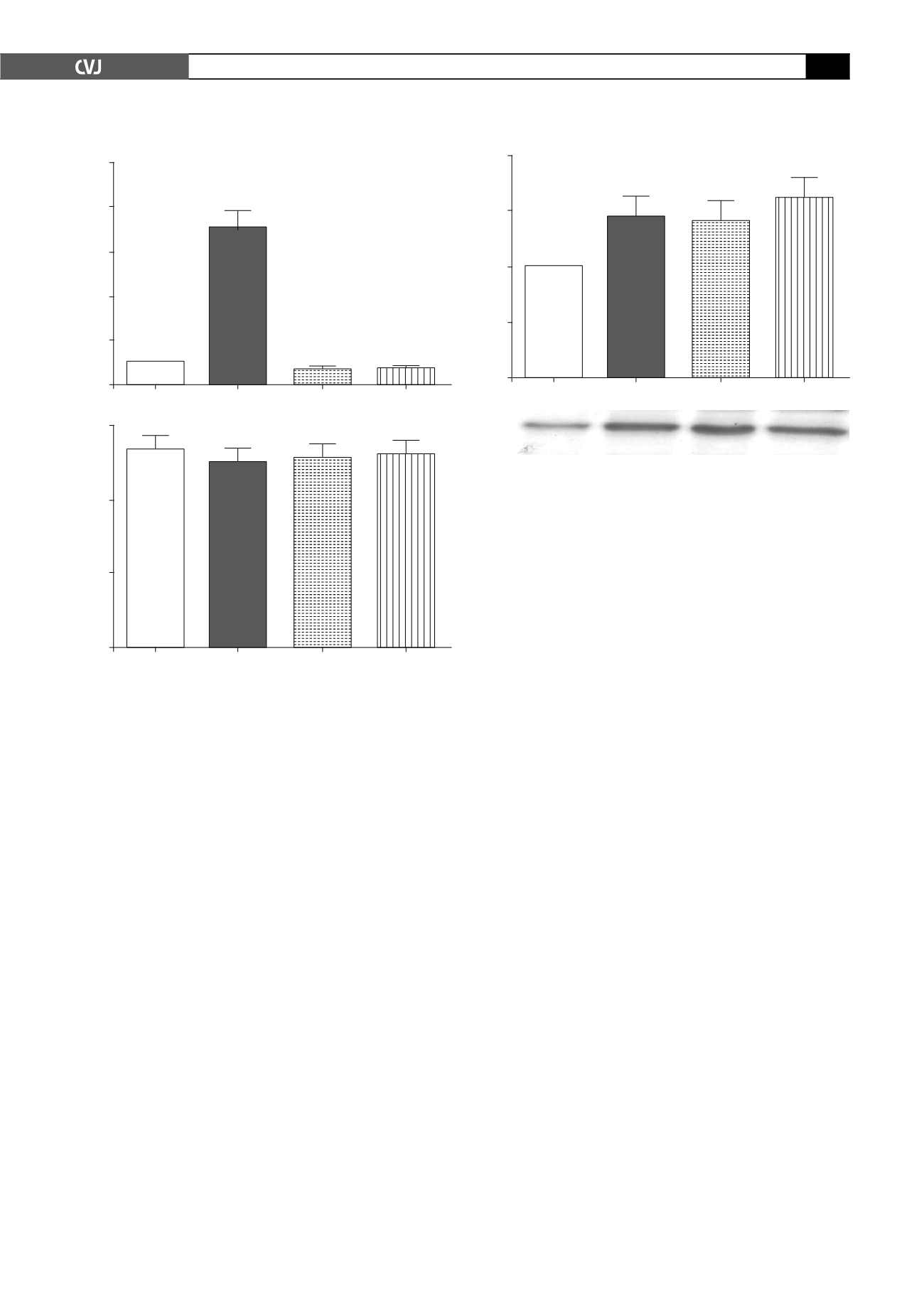
Glucose uptake
Insulin (100 nM) increased glucose uptake significantly [7.0
±
0.71-fold (
p
<
0.05)] from basal levels in the cardiomyocytes,
whereas AICAR (1 mM) diminished glucose uptake 0.6
±
0.1-fold (
p
<
0.05) from basal levels (Fig. 2A).
Fold stimulation
10
8
6
4
2
0
Basal
Insulin
AICAR
ZMP
*
*
*
#
#
Percentage
30
20
10
0
Control
Insulin
AICAR
ZMP
Fig. 2. A: Glucose uptake of cardiomyocytes as meas-
ured by the accumulation of 2-deoxy-D-[
3
H] glucose over
a 30-min incubation period after stimulation with 1 mM
AICAR, 1 mM ZMP or 100 nM insulin. Values are given as
multi-fold stimulation over a baseline of 1. B: PI staining
of the cells was performed after treatment with insulin,
AICAR and ZMP to demonstrate cell viability. All values
are expressed as mean
±
SEM (
n
=
8 individual prepara-
tions, assayed in duplicate). *
p
<
0.05 vs basal level;
#
p
<
0.05 vs insulin.
Arbitrary densitometry units
2.0
1.5
1.0
0.5
0.0
Basal
Insulin
AICAR
ZMP
**
**
**
Fig 3. A: Sarcolemmal membrane distribution of GLUT4
in basal, insulin- (100 nM), AICAR- (1 mM) and ZMP- (1
mM) treated cardiomyocytes. GLUT4 was determined via
Western blotting as described in Methods, on membrane
fractions obtained by differential centrifugation and
analysed with laser scanning densitometry. All values are
expressed as mean
±
SEM, (
n
=
5–10 individual prepara-
tions); **
p
<
0.01 vs basal. B: A representative Western
blot to show content of GLUT4 in the membrane fraction
of cardiomyocytes treated with AICAR (1 mM), ZMP (1
mM) or insulin (100 nM).
46 kDa
AICAR
–
–
+
–
ZMP
–
–
–
+
Insulin
–
+
–
–


