CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 2, March/April 2010
76
AFRICA
results clearly demonstrate that AICAR stimulation of GLUT4
translocation resulted in a protein not exposed on the outside of
the cell. In addition, it was demonstrated that insulin treatment
led to exposure of GLUT4 on the outside of the cardiomyocyte
while AICAR treatment, in accordance with the diminished
glucose uptake seen in Fig. 2A, attenuated the amount of protein
that could be recognised by the antibody on the outer surface of
the cell.
It is postulated that activation of PI-3-kinase or PKB/Akt
plays an important role in the docking and fusion of GLUT4
vesicles in insulin-stimulated glucose uptake.
31,42
However,
neither AICAR nor ZMP resulted in phosphorylation of PKB/
Akt (results not shown).
It has also been described that nitric oxide (NO) is important
in AMPK-mediated glucose uptake and GLUT4 translocation.
43
Because myocytes produce much less NO than endothelial cells,
44
we tested the effects of an NO donor, sodium nitroprusside
(SNP) in combination with AICAR on the exposure of GLUT4
on the outside of cardiomyocytes, using the flow cytometric
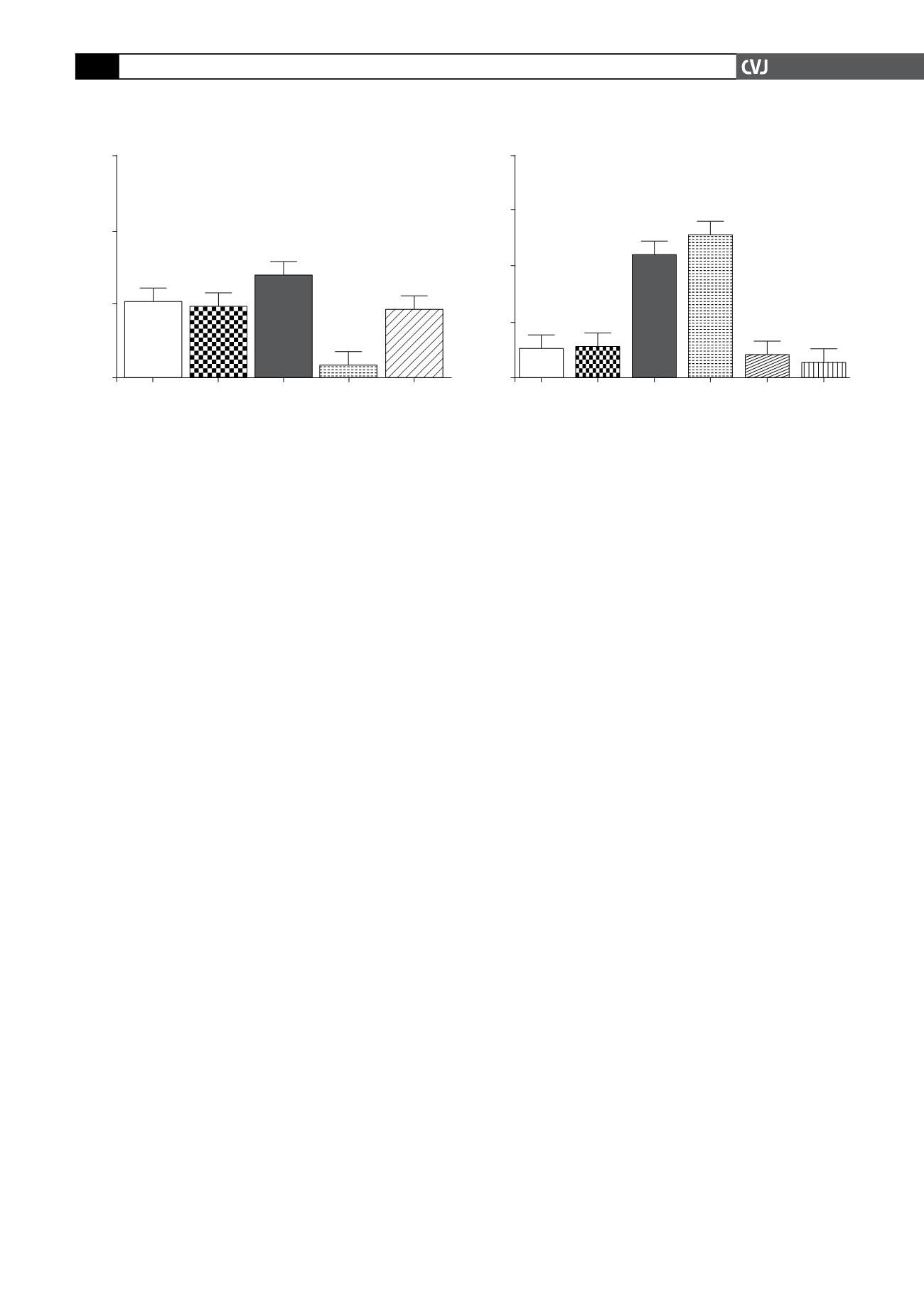
method. As shown in Fig. 4, SNP (100
µ
m) had no effect on the
number of myocytes with the exofacial loop of GLUT4 exposed
on the outside under control conditions. However, giving SNP
together with AICAR led to enhanced exposure of GLUT4 on
the outer surface of the cell. Contrary to expectation, this was not
accompanied by enhanced glucose uptake (Fig. 5).
Discussion
In this study we aimed to determine whether the pharmacological
substance, AICAR, known to activate AMPK in skeletal muscle,
also exerted similar effects on AMPK activation, glucose uptake
and GLUT4 translocation in isolated, adult ventricular cardiac
myocytes. Our results showed significantly increased AMPK
phosphorylation of Thr
172
in these cells after stimulation with
AICAR (Fig. 1), corroborating findings in EDL skeletal muscle
45
and hypothalamic cells.
46
However, Longnus
et al
.
47
were unable
to detect AMPK activation with AICAR in ventricular tissue. In
view of the conclusion of Javaux
et al
.
40
that in cardiomyocytes,
AICAR is probably not phosphorylated to ZMP, we similarly
tested the effect of ZMP and found increased phosphorylation of
Thr
172
also by this substance. Therefore, both AICAR and ZMP
can increase phosphorylation of AMPK in isolated cardiomyo-
cytes.
An increase in AMPK activity leads to stimulation of glucose
uptake in skeletal muscle.
48,49
However, the significant AMPK
phosphorylation noted in our study was not accompanied by
a concomitant increase in cardiomyocyte glucose uptake (Fig.
2A). On the contrary, there was a significant decrease in glucose
uptake seen in both the AICAR- and ZMP-treated cells. This
finding underscores the work by Jessen
et al
.
50
which showed
that basal glucose transport in AICAR-exposed animals was
significantly lower in all muscles when compared to controls
or exercised animals. Additionally, Al-Khalili
et al
.
51
found that
both chronic and short-term exposure to AICAR induced AMPK
activation in primary human skeletal myocytes but no subsequent
increase in glucose uptake.
In contrast to the above, Russell
et al
.,
14
using a slightly longer
incubation time, showed that in heart papillary muscle, incuba-
tions with AICAR increased glucose uptake almost twofold
and led to AMPK phosphorylation and GLUT4 translocation.
Papillary muscle, of course, also contains endothelial and endo-
cardial cells.
Both insulin
25,34
and AMPK
52,54
stimulate glucose uptake
by translocation of GLUT4 to the cell membrane. In view
of the findings of Russell and co-workers,
14
we quantified
GLUT4 movement to the cell membrane after various stimuli.
Fractionating cells into cytosol and sarcolemmal membranes,
insulin, AICAR and ZMP treatment resulted in significantly
more GLUT4 associated with the cell membrane (Fig. 3).
Therefore neither AICAR nor ZMP could stimulate glucose
uptake in isolated cardiomyocytes, whereas both substances
were able to phosphorylate AMPK and elicit translocation of the
GLUT4 transporter from the cytosol to the cell membrane.
The concept that GLUT4 translocation and activation to
transport glucose are two independent although interrelated
occurrences that can be separated from one another has been put
forward by Furtado
et al
.
30
To substantiate this statement, it was
demonstrated that intracellular delivery of PIP
3
results in GLUT4
Fig 5. Cardiomyocytes were stimulated as described in
Fig. 4 where after they were allowed to accumulate 2-DG
for a period of 30 min to determine glucose uptake. All
values are expressed as mean
±
SEM (
n
=
4 individual
preparations); ***
p
<
0.0001 vs basal level, SNP, AICAR
and AICAR
+
SNP.
Fold stimulation
8
6
4
2
0
Basal
SNP Insulin Insulin
+ SNP
AICAR AICAR
+ SNP
***
***
% Glut4 positive myocytes
150
100
50
0
Basal
SNP Insulin AICAR AICAR+SNP
*
Fig 4. A. Cardiomyocytes were stimulated as described
in Methods, with 1 mM AICAR, 100 nM insulin and 100
µ
M SNP. GLUT4 protein was visualised with Alexa Fluor
488 coupled to an antibody directed against the exofa-
cial loop of the protein. Positive cells were defined by a
fixed gate and expressed as a percentage of the total cell
population. All values are expressed as mean
±
SEM (
n
=
4 individual preparations); *
p
<
0.05 vs basal level.


