CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 6, November/December 2010
AFRICA
335
Is there a unifying pathogenetic mechanism
for PAH?
The complexity of receptor activation, signalling molecules and
downstream pathways with cross-talk at different levels between
these makes it unfortunately difficult to pinpoint a precise mech-
anism for PAH (Fig. 2). Nevertheless, the discovery of a loss-of-
function mutation in bone morphogenetic receptor II (BMPR2),
a member of the TGF-
b
superfamily, in 20 to 30% of patients
with idiopathic PAH (IPAH) and 60% of patients with familial
PAH immediately paved the way for elucidating the pathobiology
of the disease.
6,7
TGF-
b
receptors together with their ligands (proteins that
bind to the receptor) control diverse processes involved in vascu-
lar remodelling, including among others, cell proliferation and
apoptosis, cellular differentiation, and collagen and extracellular
matrix turnover. These are all processes that are fundamentally
involved in PAH but the precise link between the genotype and
the expression of the pulmonary hypertensive phenotype remain
elusive.
According to the current hypothesis, a loss-of-function
mutation in the BMPR2 receptor results in an imbalance in the
equilibrium between the opposing effects of TGF-
b
and BMP
signalling, which in the smooth muscle cell (SMC) favours a pro-
proliferative and anti-apoptotic response but in the case of the
endothelial cell (EC), has an anti-proliferative and pro-apoptotic
effect (Fig. 3). Although the reason for the contrasting effect of
BMP signalling on the SMC and EC remain unknown, the model
provides compelling evidence for the pathobiological underpin-
nings of PAH. Furthermore, emergence of apoptosis-resistant
clones of ECs may account for unregulated proliferation and the
formation of plexogenic lesions.
8
How is PH best defined, classified and
investigated?
Although PAH is optimally defined on pathology, this is rarely
possible except post-mortem. From a haemodynamic perspec-
tive, pulmonary vascular resistance is the best measure of the
resistance of the pulmonary circulation to flow but this in general
requires invasive cardiac catheterisation, which is inappropriate
as a screening test. Therefore an estimate of pulmonary artery
pressure forms the starting point for diagnosis of PH.
The time-honoured clinical methods, including a left paraster-
nal heave, palpable P2, pulmonary ejection click and narrow
A2-P2 split are important, as are the ECG showing P-pulmonale,
right-axis deviation and a tall R in V1 and chest X-ray with
characteristic dilatation of the proximal pulmonary arteries and
attenuation of distal third of the vasculature. However, echo-
Doppler is an easily available, inexpensive and non-invasive
way of obtaining a comprehensive assessment of pulmonary
haemodynamics and is recommended as the initial investigation
of choice in most guidelines.
2,3
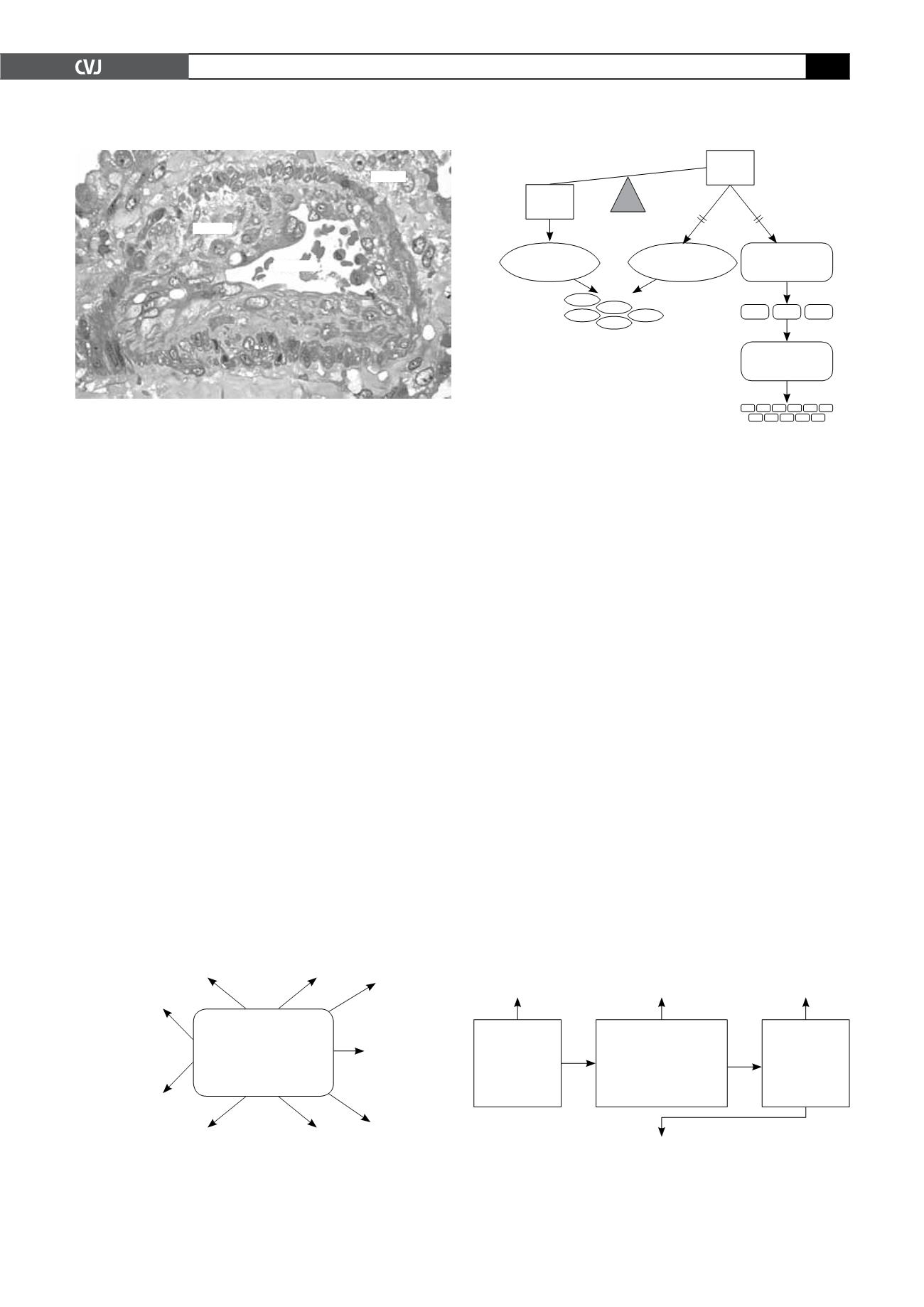
Fig. 1. Typical plexiform lesion showing marked intimal
hyperplasia due to disordered enthothelial cell prolifera-
tion and obliteration of the lumen.
Intima
Lumen
Media
Fig. 2. Pathobiological mechanisms in pulmonary arterial
hypertension.
Apoptosis
Proteolysis
Platelet
activity and
thrombosis
Endothelial cell
differentiation
and migration
Inflammation
Chemotaxis
Collagen and
extracellular
matrix
deposition
Smooth
muscle cell
differentiation
and proliferation
Expression of
vasodilator and
vasoconstrictor
peptides, and
growth factors
ET-1, endothelin 1; 5-HT, serotonin; Kv, potassium channel; PGI
2
, prostacyclin; TXA
2
,
thromboxane A2; VIP, vasoactive intestinal peptide
5-HT
VIP
Kv
EMPs
TGF-
b
MMPs
Elastase
VEGF
PDGF
EGF
FGF
ET-1
NO
PG1
2
TXA
2
Left heart
pathology and
shunts
Auto-immune disease;
HIV; polycythaemia;
sickle cell disease,
cirrhosis; hypoventilation
Lung
abnormality;
thrombo-emoblic
disease
Echo
suggestive
of pulmonary
hypertension
Blood screen
indicating auto-
antibodies; HIV,
haemoglobin, liver
functions, arterial
blood gas (ABG)
Chest X-ray;
V/Q scan; CT;
pulmonary
angiogram;
lung function
Cardiac catheterisation
Vascular testing
Pulmonary angiography
Fig. 4. Recommended sequence of investigations in
patients with pulmonary hypertension.
Fig. 3. Unifying mechanism for smooth muscle cell prolif-
eration, endothelial loss and proliferation in patients with
loss-of-function mutation in BMPR2.
SMC
SMC
proliferation
EC loss/
dysfunction
EC hyperplasia
EC
=
endothelial cell;
SMC
=
smooth muscle cell
Mutant
BMPR2
TG-
b
Apoptosis
resistant cell
SMC
Pro-proliferative
anti-apoptotic
Anti-proliferative
pro-apoptotic
Proliferative
anti-apoptotic
EC


