
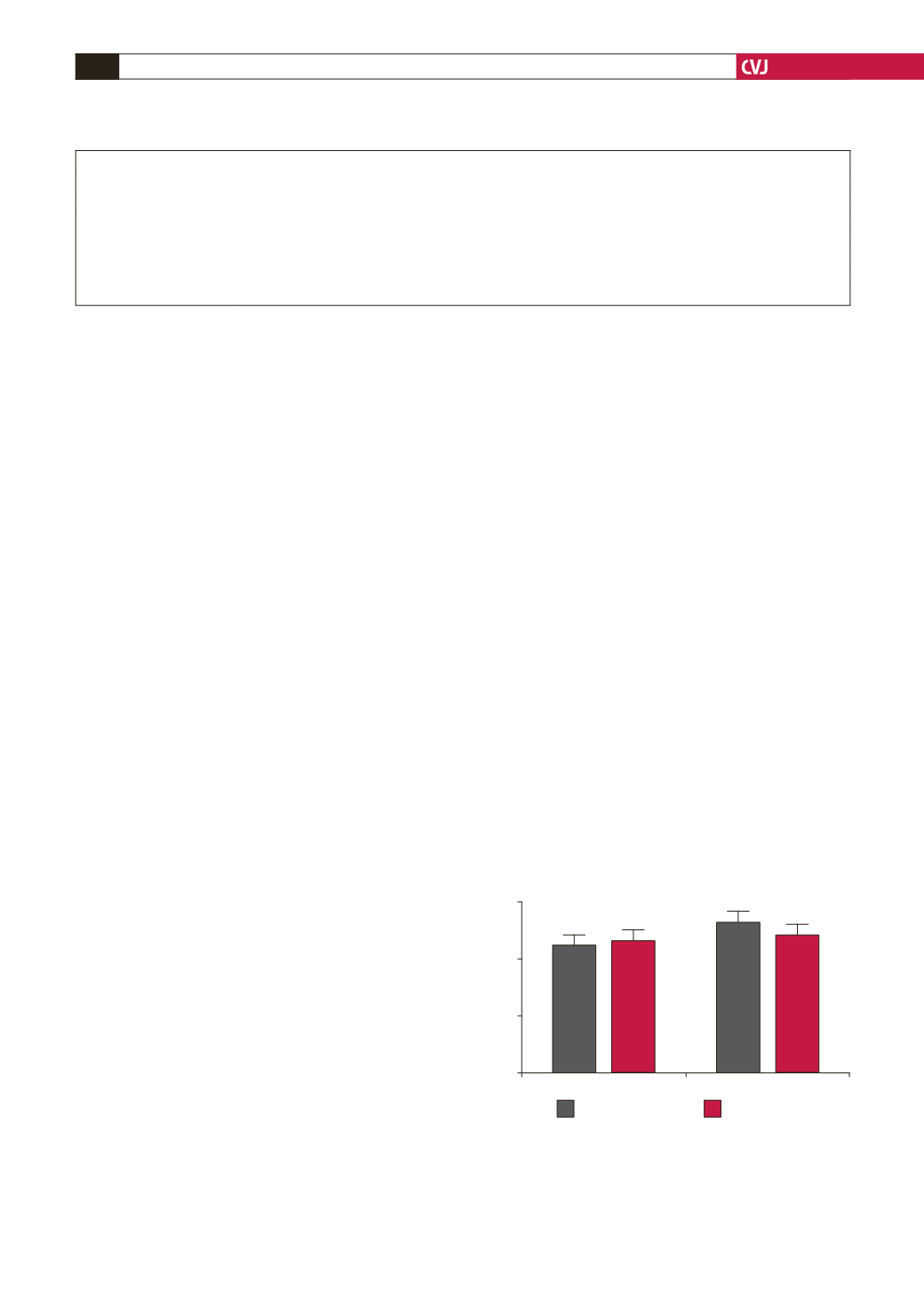
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 2, March 2013
12
AFRICA
Western blotting
Frozen tissues were pulverised with a liquid nitrogen pre-cooled
mortar and pestle and then extracted in lysis buffer containing
in mM: Tris-HCl 20 (pH 7.5), EGTA 1, EDTA 1, NaCl 150,
Na
2
VO
3
1, beta-glycerophosphate 1, sodium-pyrophosphate 2.5,
PMSF 0.3, Triton X-100 1% (v/v) plus 10 µg/ml leupeptin and
aprotinin, respectively, using a Polytron PT10 homogeniser, 2 × 4
sec, at setting 4. Lysates were cleared from particulate matter by
centrifuging for 15 min at 14 000 rpm in a microfuge (Eppendorf
Mini-spin plus, Hamburg, Germany) and the protein content was
determined by the method of Bradford.
16
Samples were diluted
in Laemmli sample buffer, boiled for 5 min and stored at –80°C.
Equal amounts of cytosolic proteins were separated on a SDS
poly-acrylamide gel and electro-transferred to Immobilon
TM
-P
PVDF membranes. Transfer and equal loading of proteins was
determined with Ponceau red reversible stain. The membranes
were blocked for two hours in Tris-buffered saline (TBS)
containing 0.1% Tween-20 and 5% non-fat milk powder and
incubated overnight in the primary antibodies (diluted in
TBS–Tween according to the manufacturer’s instructions). The
following antibodies from cell signalling were used: insulin
receptor beta-subunit, phospho-PI3K P85 (Tyr458), total and
phospho-PTEN (Ser380/Thr382/383), total and phospho-PKB/
Akt (Ser473), Glut 1 and Glut 4.
Blots were stripped using a 5-min incubation in 2% NaOH
after washing in distilled water and re-probed with a beta-tubulin
antibody to confirm equal loading. Bands were visualised using
the ECL detection system and quantified by laser-scanning
densitometry with suitable software (Silk Scientific Inc, USA).
For comparison purposes, total pixels of bands were expressed as
a ratio of the mean of the controls on the same blot.
Statistical analyses
Data are presented as mean
±
SEM and were analysed using
either a one-way or two-way ANOVA followed by a Bonferroni
post-hoc
test for differences between groups. The blood pressure
effects were analysed using a repeated-measures two-way
ANOVA. Statistical significance was set at
p
<
0.05.
Results
After the 16-week diet animals from model 1 (DIO) presented
with significantly increased body- and intra-peritoneal fat
weight (Table 1). As summarised in Table 1, these animals had
significantly elevated blood glucose and insulin levels, leading to
an increased homeostatic model assessment of insulin resistance
index (HOMA-IR), indicative of whole-body insulin resistance.
In neither control nor DIO animals did the treatment with
P
glandulosa
have any effect on the body weight or the intra-
peritoneal fat weight of the animals. After treatment of the DIO
animals with
P
glandulosa
, the blood glucose levels were no
longer significantly elevated compared to the treated controls but
the HOMA-IR was still significantly higher. However, as shown
in Fig. 1, the two-hour blood glucose values after intra-peritoneal
glucose tolerance analyses were significantly lower in the treated
DIO animals, underscoring a slight effect on blood glucose
handling, as previously reported.
8
Infarct size
After 16 weeks of the obesity-inducing diet, the
ex
vivo
perfused
hearts of the DIO animals presented with significantly larger
infarct sizes, calculated as percentage of the area at risk, than
the hearts from the control animals (DIO 49.48
±
3.25 vs control
40.62
±
2.21%,
p
<
0.05,
n
=
17 per group). The area at risk did
not differ between the groups and averaged 54.13
±
2.21%.
An eight-week treatment regime with
P
glandulosa
in
conjunction with the diet significantly improved the ability of the
hearts to withstand a period of ischaemia, and smaller infarcts
developed. There was no significant effect in the hearts from
control rats (Fig. 2). Two-way ANOVA indicated a significant
effect of the treatment on infarct size (
p
<
0.01).
To confirm these results and rule out any effect of insulin
levels on the cardioprotective role of
P
glandulosa
, we used a
mouse model with a conditional ablation of the insulin receptor
in cardiomyocytes.
14
Subjecting these animals and their normal
C57Bl6 littermates to
ex
vivo
perfusion and NICA, followed by
reperfusion, we found that the hearts of both control and CIRKO
mice were protected by the
P
glandulosa
treatment. This was
Fig. 1. DIO or control chow-fed rats for 16 weeks with
P glandulosa
treatment for the last eight weeks were
subjected to intra-peritoneal glucose-tolerance testing
after an 18-hour fast. Blood was collected by tail prick
and analysed over a 120-min period using a commercial
glucometer. Data given are the 120-min values. *
p
<
0.05
vs control and DIO plus treatment,
n
=
6 per group.
6
4
2
0
Control
DIO
Clucose concentration (mmol/l)
minus treatment
plus treatment
*
TABLE 1. BIOMETRIC DATA – MODEL 1: DIO
Control
Control +
P glandulosa
DIO
DIO +
P glandulosa
Weight
433.7
±
9.3
438.6
±
9.3
507.7
±
22.9***
534.3
±
11.7***
Intra-peritoneal fat
18
±
2.7
11
±
1.8
28.0
±
1.74***
34
±
1.4***
Blood glucose (mmol/l)
5.42
±
0.17
5.4
±
0.18
6.4
±
0.17*
5.6
±
0.19
Serum insulin (
µ
U/ml)
17.12
±
0.8
14.07
±
1.50
34.33
±
9.06*
35.93
±
10.21*
HOMA-IR
4.73
±
0.71
3.40
±
0.40
8.96
±
2.65*
7.88
±
3.30*
*
p
<
0.05 vs the respective control; ***
p
<
0.001 vs the respective control. Analysis by two-way ANOVA,
n
=
6 per group.



















