
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 2, March/April 2019
AFRICA
111
but impaired long-term altitude acclimatisation.
24
This could be
explained by the higher sympathetic activation found in smokers
in our study. However, contrary to previous studies focused on
autonomic control and oxygen saturation in smokers at high
altitude, we used normobaric hypoxia in an indoor environment,
with a constant temperature, higher than can be expected at high
altitude, which are all factors that could affect the results.
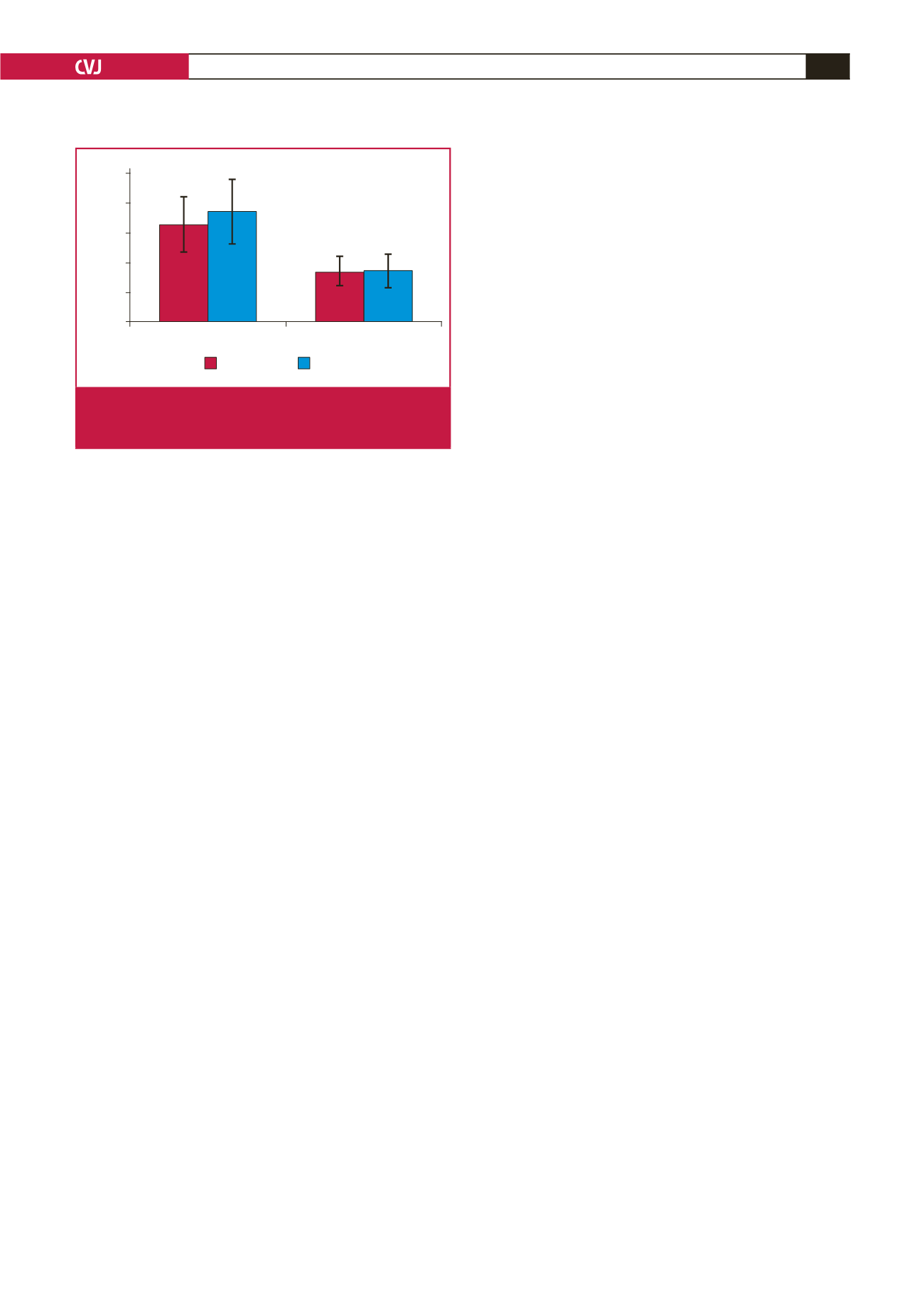
The shift fromhypoxic to normoxic conditions in non-smokers
was associated with a sudden increase of SpO
2
and overall
variability (SDNN), caused by parasympathetic activation
(RMSSD, lnHF) (Fig. 2), which is normally observed in the
recovery period in subjects with good autonomic control, such as
athletes.
25
Conversely, there was no change in HRV parameters in
smokers and the LF/HF index even increased, which was evident
in demonstrating the absence of parasympathetic response when
the hypoxic stimulus was removed. The absence of autonomic
response was most probably a result of autonomic dysfunction
in the smokers, which has previously been described in the
literature.
9,26
There is little data about the changes in activity of the ANS
when shifting from hypoxic to normoxic environments. Since
transition from hypoxic to normoxic conditions can induce
PSNS activation, which was not observed in the smokers,
our hypoxic provocation protocol could be applied in clinical
practice as a test for early detection of autonomic dysfunction in
smokers, even before the appearance of clinical signs.
Some study limitations of our research should be considered.
We did not record the respiratory rate and tidal volume, therefore
we do not have information on ventilation, which is known
to affect HRV parameters.
15
However, the peak frequency of
HF, which is suggested as an indirect index of respiratory rate,
showed no changes during the different periods of the protocol.
18
We therefore assumed that ventilation of the examined subjects
was stable.
The sample of the study was not large enough to produce
more explicit results. Increasing the number of enrolled subjects
would potentially decrease the impact of confounding factors
affecting HRV. Additionally, even though we tried to control the
environmental factors that can affect HRV results, there were
more uncontrollable, non-quantifiable factors such as stress and
quality of sleep that could have had an impact on the ANS and
should be considered.
Conclusion
The results of our study show that smoking impaired the
autonomic modulation in ‘healthy’ young smokers and led
to a decreased HRV even before the appearance of any
subjective clinical signs and symptoms. During acute exposure
to exogenous hypoxia, smokers had higher SpO
2
but lower
HRV parameters – RMSSD, lnLF, lnHF and Poincaré plot
standard deviations SD1, SD2 and SD1/SD2 index. The data
suggest that smokers have altered autonomic regulation under
hypoxic conditions: diminished parasympathetic activity and
sympathetic domination despite having higher SpO
2
. Transition
from hypoxic to normoxic conditions leads to an increase
in PSNS activity, which was observed in only the group of
non-smokers. Therefore, a hypoxic exposure test could be used
in clinical practice for early detection of autonomic dysfunction
in smokers, because their parasympathetic reactivation is blunted
when shifting from hypoxic to normoxic ambient conditions, as
measured by HRV.
The study was carried out with a grant under Project no
BG05M2OP001-2.009-0025, ‘Doctoral training at MU-Plovdiv for
Competence, Creativity, Originality, Realization and Academism in Science
and Technology – 2 (DOCTORANT – 2)’, funded under the operational
programme ‘Science and Education for Smart Growth’, and co-funded by the
Structural and Investment Funds of the EU.
References
1.
Zatonski W, Przewozniak K, Sulkowska U, West R, Wojtyla A. Tobacco
smoking in countries of the European Union.
Ann Agric Environ Med
2012;
19
(4): 181–192.
2.
Modala S, Ahmed QR, Sau SK. Effect on autonomic nervous system in
smokers and non-smokers – a comparison study.
Natl J Med Allied Sci
2012;
1
(1): 25–28 .
3.
Papathanasiou G, Mamali A, Papafloratos S, Zerva E. Effects of smok-
ing on cardiovascular function: the role of nicotine and carbon monox-
ide.
Health Sci J
2014;
8
(2): 274–290.
4.
Ferreira M, Zanesco A. Heart rate variability as important approach for
assessment autonomic modulation.
Motriz: J Phys Ed
2016;
22
(2): 3–8.
5.
Kang JH, Kim JK, Hong SH, Lee CH, Choi BY. Heart rate variabil-
ity for quantification of autonomic dysfunction in fibromyalgia.
Ann
Rehabil Med
2016;
40
(2): 301–309.
6.
Sgoifo A, Carnevali L, Alfonso Mde L, Amore M. Autonomic dyfunc-
tion and heart rate variability in depression.
Stress
2015;
18
(3): 343–352.
7.
Manzano BM, Vanderlei LC, Ramos EM, Ramos D. Acute effects of
smoking on autonomic modulation: analysis by Poincare plot.
Arq Bras
Cardiol
2011;
96
(2): 154–160.
8.
Behera JK, Sood S, Gupta R, Kumar N, Singh M, Gupta A. Assessing
autonomic function in smokers.
Australas Med J
2010;
3
(11): 712–715.
9.
Ferdouse M, Ferdousi S. Autonomic dysfunction in current cigarette
smokers assessed by time series analysis of heart rate variability.
J
Bangladesh Soc Physiol
2013;
8
(2): 84–88.
10. Middlekauff HR, Park J, Moheimani RS. Adverse effects of cigarette
and noncigarette smoke exposure on the autonomic nervous system.
J
Am Coll Cardiol
2014;
64
(16): 1740–1750.
11. Papathanasiou G, Georgakopoulos D, Papageorgiou E, Zerva E,
Michalis L, Kalfakakou V, Evangelou A. Effects of smoking on heart
rate at rest and during exercise, and on heart rate recovery in young
adults.
Hellenic J Cardiol
2013;
54
(3): 168–177.
12. Najem B, Houssiere A, Pathak A, Jansen C, Lemogoum D, Xhaet O,
Groups
Non-smokers
Smokers
RMSSD (ms)
100
80
60
40
20
0
Hypoxia
Normoxia
Error bars: 95% CI
p
= 0.011
p
= 0.914
Fig. 2.
Comparison of RMSSD between non-smokers and
smokers under hypoxic and post-hypoxic (normoxic)
ambient conditions.

















