
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 6, November/December 2019
AFRICA
353
longitudinal study examining changes in lifestyle and focusing
on low-, middle- and high-income countries.
16
In the South
African leg of the PURE study, performed in the North West
Province, 2 010 participants were randomly recruited from
Potchefstroom (urban
n
=
1 004) and Ganyesa (rural
n
=
1 006).
Data were collected on three occasions, at baseline in 2005, and
follow up in 2010 and 2015. Black individuals older than 35 years
were invited to take part in the study and were fully informed,
procedures were explained and they gave written informed
consent. Pregnant and lactating women were excluded
During the 2010 follow up, 1 288 subjects participated in
the study, while 221 died and 501 were lost to follow up. In the
second follow up in 2015, 926 returned for follow up and 127
deaths were recorded, while 307 did not return for follow up.
The attrition level of participants from baseline (2005:
n
=
2 005)
to follow up (2015:
n
=
926) is similar to previous longitudinal
studies as a result of refusal to take part, relocation to other
locations of the country, ill health of older individuals and
death.
In the current study, at baseline in 2005, 320 of the total study
population of 2 010 were newly identified with HIV, and at the
10-year follow up, 117 were retained. The 320 newly identified
HIV-infected participants were matched with uninfected controls
according to age, gender, BMI and locality at baseline. For
this longitudinal study, we followed 117 HIV-infected and 131
uninfected participants who participated in the 10-year follow-
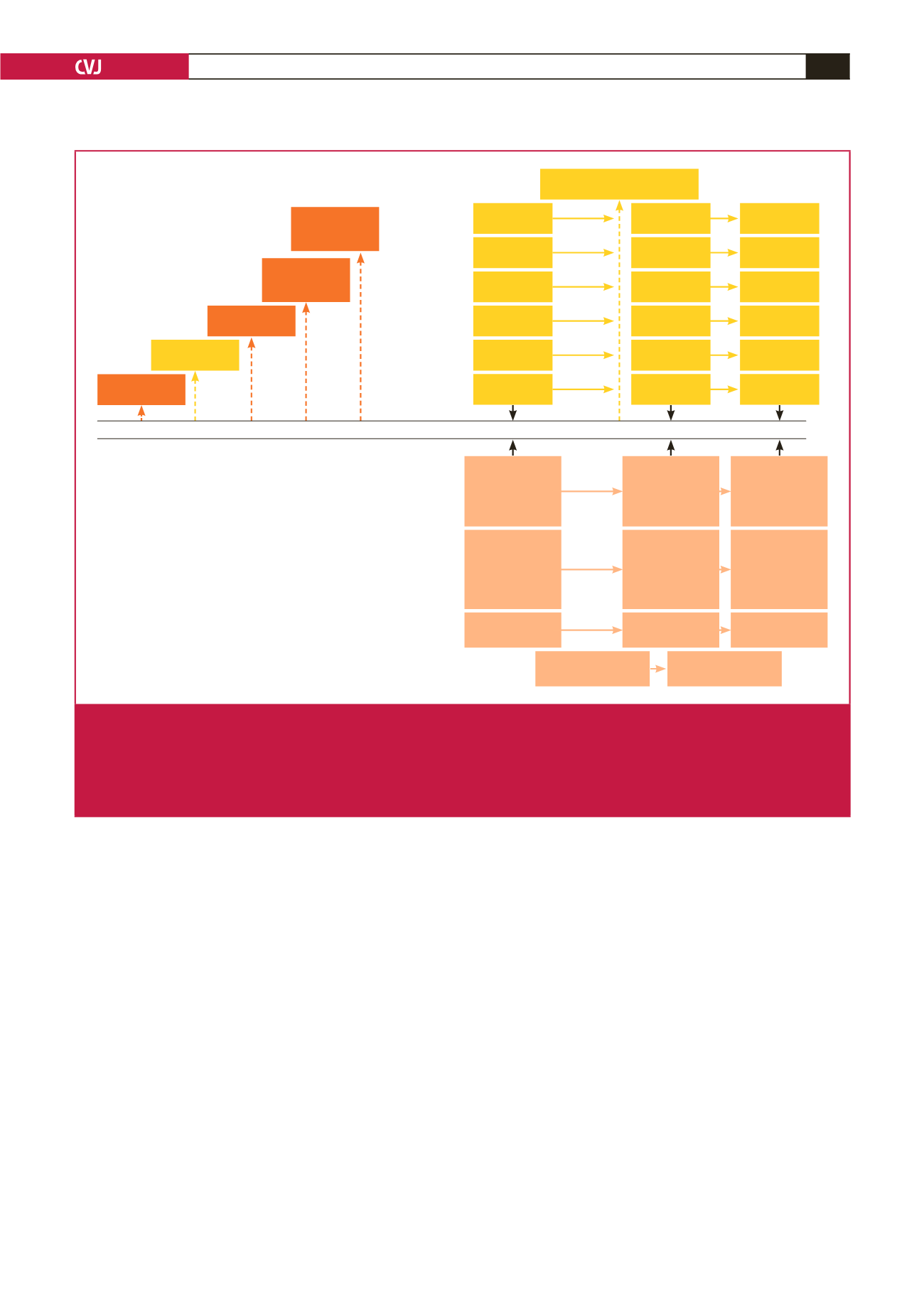
up data collection. The study population is outlined in Fig. 2.
In 2005, the participants who were newly diagnosed with
HIV and were ART-naïve were referred for follow up and
CD4 cell count determination to initiate ART according to
the guidelines of the South African Department of Health.
Five years later (2010), 70 were on ART, which increased to 77
in 2015. ART comprised two nucleoside reverse transcriptase
inhibitors (NRTIs) and one non-nucleoside reverse transcriptase
inhibitor (NNRTI) (Fig. 1).
In South Africa, the ART guidelines changed over the
10 years of follow up. In 2004, when ART was introduced,
stavudine was the backbone of the ART regimen. However, it
was phased out due to its association with lipodystrophy and
was replaced with tenofovir in 2010.
17
Fixed-dose combination
was introduced in 2012,
17
and a ‘test-and-treat’ programme was
implemented in September 2016.
5
3.4 million
1.3 million
1.1 million
1st HIV
antibody test
Early CVD cases
in HIV+: Western
countries
HIV+:
n
= 320
New HIV infections:
100%
HIV prevalence:
15.9%
ART users
0%
Mean CD4 cell count:
314 cells/mm
3
Mortality:
HIV+: 24% vs HIV–: 0.9%
Mortality:
HIV+: 0% vs HIV–: 0%
HIV+:
n
= 163
New HIV infections:
6.3%
HIV prevalence:
12.7%
ART users
70/161 (46.4%)*
d4T/3TC/EFV or
AZT/3TC/EFV
TDF/3TC/FTC or EFV/
NVP
Mean CD4 cell count:
302 cells/mm
3
HIV+:
n
= 117
New HIV infections:
6.2%
HIV prevalence:
12.7%
ART users
77/91 (84.6%)
#
FDC
Mean CD4 cell count:
507 cells/mm
3
Global HIV
programme
launched by WHO
1st HIV diagnosis:
America
1
1st HIV
diagnosis: SA
Early CVD cases in HIV+: SA
≤
500 cells/mm
3
<
300 cells/mm
3
<
200 cells/mm
3
FDC
TDF + 3TC/FTC +
EFV/NVP
12.5%
(6.19 million)
12.1%
(6.12 million)
d4T + 3TC + EFV
11.5%
(5.35 million)
1.06%
1.46%
1.86%
18.2%
18.3%
17.9%
ART use: SA
4,5
Global and South African HIV timeline
PURE study, North West Province, South Africa
CD4 cut-off
20
1st line regime
20
SA: HIV prevalence
6
SA: HIV incidence
6
HIV: 15–49 yrs
6
1981
1982
1985
1987
1990
1995
2000
2005
2007
2008
2010
2013
2015
Fig. 1.
Selected events in tracking HIV, ART and CVD. HIV, human immunodeficiency virus; ART, antiretroviral therapy; FDC, fixed-
dose combination; d4T, stavudine; TDF, tenofovir; 3TC, emtricitabine; EFV, efivarenz; FTC, lamivudine; NVP, nevirapine; AZT,
zidovudine; SA, South Africa; WHO, World Health Organisation; CVD, cardiovascular disease; PURE, Prospective Urban
and Rural Epidemiology study; HIV+, HIV infected; HIV–, HIV uninfected; vs, versus. *Out of the 163 HIV-infected individuals
who were followed in 2010, information on the use of ART was available for 151 participants.
#
Out of the 117 HIV-infected
individuals who were followed in 2015, information on ART use was available for 91 participants.



















