
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 6, November/December 2019
354
AFRICA
The PURE study was approved by the Health Research
Ethics Committee of the North-West University, South Africa.
The study protocol and procedures were explained to the
participants in their home language (Setswana) and they gave
written informed consent.
Questionnaires were used to collect data on demographic
information, current health status, medical and family history,
medication as well as tobacco and alcohol use. Standardised
procedures were used for anthropometric measurements,
including height, weight and waist and hip circumferences.
18
Systolic blood pressure (SBP) and diastolic blood pressure
(DBP) measurements were taken, in duplicate at an interval of
five minutes on the right arm while in a sitting position. The
validated OMRON HEM-757 device was used at baseline and
at the 2010 follow up while the OMRON M6 device (Omron
Healthcare, Kyoto, Japan) was used during the 2015 follow up.
Venous blood samples were collected from the participants
after fasting for at least eight hours. Serum and plasma were
prepared and along with spot urine samples, stored at –80°C.
Glucose levels from fluoride plasma samples were determined
using the Vitros DT6011 chemistry analyser (Ortho-Clinical
Diagnostics, Rochester, New York, USA) in 2005 and the
Cobas Integra 400 plus (Roche, Indianapolis, IN) at follow up.
Glycated haemoglobin (HbA
1c
) was determined using the D-10
haemoglobin testing system from Bio-Rad (Hercules, California,
USA).
Serum samples were used to analyse levels of high-
sensitivity C-reactive protein (hsCRP), total cholesterol (TC),
triglycerides (TG), high-density lipoprotein cholesterol (HDL-
C),
γ
-glutamyltransferase (GGT), aspartate transaminase
(AST), alanine transaminase (ALT) and creatinine using a
Konelab20iTM auto-analyser (Thermo Fisher Scientific Oy,
Vantaa, Finland) in 2005 and a Cobas Integra 400 plus auto-
analyser (Roche, Indianapolis, IN) in 2010 and 2015. Low-density
lipoprotein cholesterol (LDL-C) levels were calculated.
19
Estimated glomerular filtration rate (eGFR) was determined
using the chronic kidney disease epidemiology collaboration
(CKD-EPI) equation in ml/min/1.73 m
2
.
20
Urinary creatinine and
albumin levels were analysed using the Cobas Integra 400 plus
(Roche, Basel, Switzerland) in 2005 and 2015, and the urinary
albumin-to-creatinine ratio (uACR) was calculated.
The HIV status of all participants was determined from
whole blood finger-prick using the first response rapid HIV card
test (Premier Medical Corporation Limited, Daman, India).
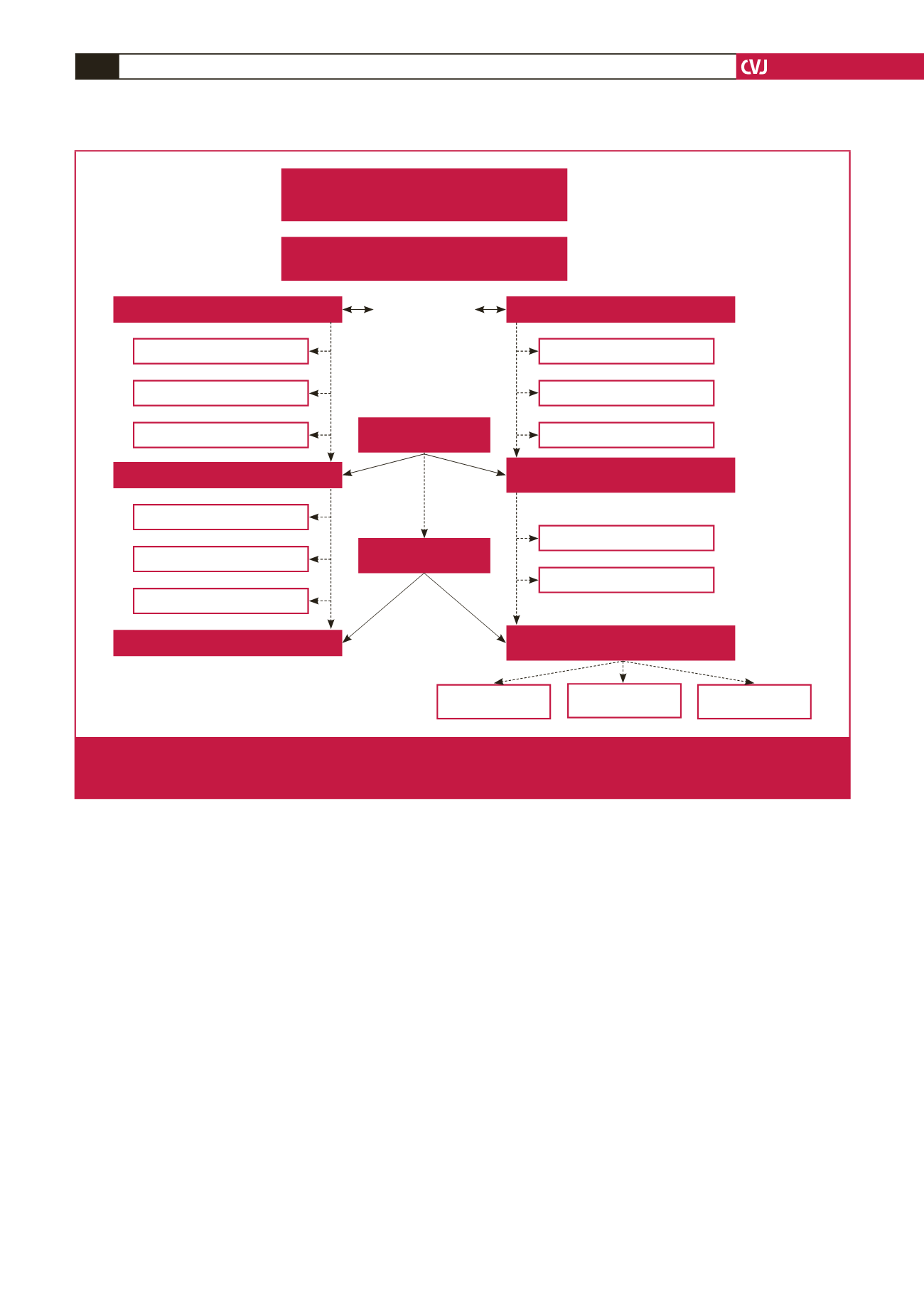
PURE study
Baseline data collection in 2005,
n
=
2010
HIV uninfected
n
=
1688 & HIV infected
n
=
322
#
For this study
Matched HIV uninfected & infected at baseline,
n
=
640
HIV uninfected (controls),
n
=
320
HIV uninfected,
n
=
192
HIV uninfected,
n
=
120
+
11*,
n
=
131
Lack of reparticipation,
n
=
112
Deceased,
n
=
3
Lack of reparticipation,
n
=
65
Newly HIV infected,
n
=
13
Deceased,
n
=
0
Newly HIV infected,
n
=
7
HIV uninfected (newly identified),
n
=
320
HIV uninfected
n
=
150
+
13 newly infected,
n
=
163
HIV infected
n
=
99
+
7 newly infected
+
11*,
n
=
117
Lack of reparticipation,
n
=
93
Lack of reparticipation,
n
=
64
HIV ART naive
n
=
14
HIV infected on ART
n
=
77
No ART information
n
=
26
Deceased,
n
=
77
Deceased,
n
=
0
Newly HIV infected,
n
=
13
First follow-up 2010
n
=
355
Second follow-up 2015
n
=
248
Matched for age,
gender, BMI, locality
Fig. 2.
Outline of the study population. ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus;
n
,
number of participants; PURE, Prospective Urban and Rural Epidemiology study.
#
Two of the participants were excluded due
to incomplete data. *Participants who were not followed up in 2010 but in 2015: 11 HIV-infected and 11 uninfected participants.



















