
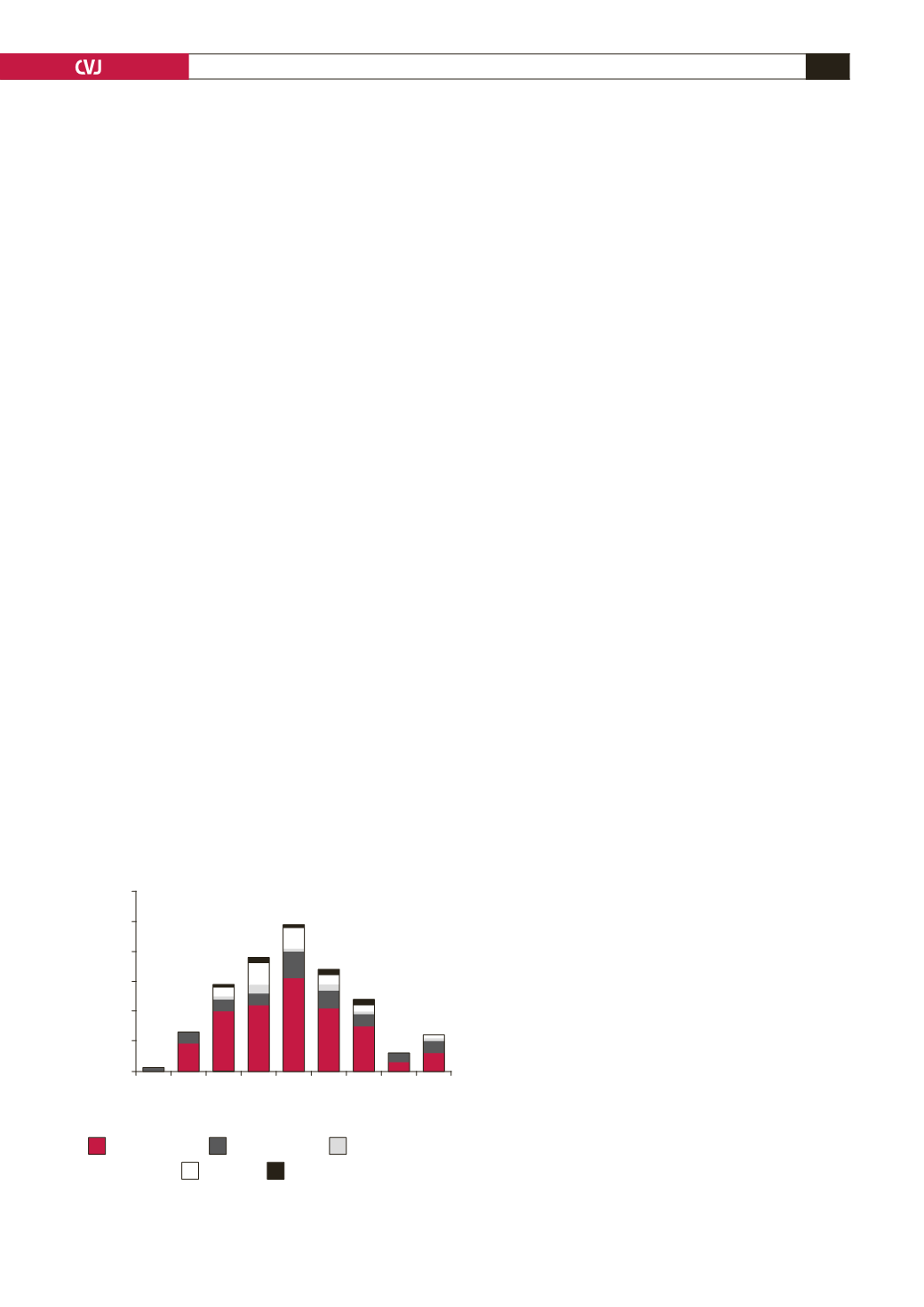
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 2, March 2013
AFRICA
39
plasma triglyceride levels pre-HAART and prior to developing
AIDS.
48
Both traditional and non-traditional risk factors therefore
appear to contribute to atherosclerotic disease in HIV-infected
patients. Those on HAART, particularly protease inhibitors,
develop a myriad of class- and non-class-specific metabolic
effects on lipid profiles, glucose levels, insulin sensitivity and
anthropometric body changes characteristic of lipodystrophy.
Untreated HIV infection may also have a paradoxical overall
effect on cardiovascular disease and thereby reduce the risk of
ischaemic heart disease because of severe and progressive weight
loss, wasting syndrome, hypotension resulting from chronic
gastroenteritis, hypoadrenalism and shortened life expectancy
associated with advanced AIDS.
Despite the scarcity of data from SSA, there are some
indications of overall excess CVD risk factors in HIV-infected
patients. Situation analysis in 2008 of 501 HIV-infected patients
from Botswana using the database of the Botswana Medical Aid
Scheme combined with data from the Centre for Chronic Diseases
revealed impressive clustering of hypertension, dyslipidaemia,
obesity, dysglycaemia and smoking (Fig. 2). The peak age range
for the occurrence of CVD risk factors was about a decade after
the peak age for HIV infection in Botswana.
Given the difficulty of determining whether the observed
increase in CVD risks were due to HIV itself, treatment with
HAART or merely a factor of improved longevity, it would be
ideal to perform case–control studies on the prevalence of CVD
risk factors and the prevalence of arteriosclerotic cardiovascular
endpoints such as IHD, stroke, and peripheral arterial disease in
HIV-infected versus age- and gender-matched non-HIV-infected
individuals. Also, a comparison of pre-HAART and on-HAART
HIV-infected patients would shed light on this grey area. It
is important to remember that the enormous impact of HIV/
AIDS does not appear to have diminished the impact of chronic
cardiovascular diseases on mortality in SSA.
49
Reports on IHD in SSA
There are a few scattered reports of IHD in SSA. Kengne and
colleagues
50
collated a total of 356 cases of SSA patients with
coronary heart disease (CHD) from four selected countries
(Ghana, Cameroon, Senegal and Kenya). They reported a high
prevalence of CHD risk factors, which was not surprising in this
selected population of patients with established CHD. Males
outnumbered females by ratios ranging from 1.3:1 to 6:1, with
hypertension in up to two-thirds of the patients. The report
highlighted the fact that IHD was by no means rare in these
African populations.
The African arm of the INTERHEART study showed
that dyslipidaemia, abdominal obesity and tobacco use
accounted for greater population-attributable risk in the overall
African population, whereas hypertension and diabetes were
less prominent risk factors.
51
However, in black Africans,
dyslipidaemia was followed by hypertension, abdominal obesity,
diabetes and then tobacco use.
The INTERHEART African study cast doubt on the notion
of protective lipid profiles in blacks, as one reason for implicitly
low IHD prevalence in Africa. High HDL cholesterol levels in
black Africans might be dysfunctional and less protective than
generally believed. However, the findings of the INTERHEART
African study were at slight variance with reports by Ezzati and
colleagues who showed that hypertension, low intake of fruits
and vegetables and physical inactivity accounted for population-
attributable fractions for ischaemic heart disease mortality of
43, 25 and 20%, respectively, in the Africa region. These were
all above the population-attributable fraction of 15% for high
cholesterol.
52
Limitations in diagnostic evaluation of patients with possible
IHD might explain, at least in part, the apparent rarity of IHD in
SSA. This is illustrated by the study on black South Africans by
Joubert and colleagues using data from the Medical University of
South Africa (MEDUNSA) stroke data bank. The study showed
increased prevalence of CHD with improved diagnostic tools.
53
History of angina pectoris or myocardial infarction using
the Rose questionnaire yielded a prevalence of only 0.7% in
741 black patients with stroke, 71% of whom had cerebral
infarction. Resting 12-lead electrocardiography was analysed for
the presence of poor R-wave progression in the precordial leads,
the presence of pathological Q waves and ST–T wave changes
using the Minnesota code in 555 stroke patients, 72% of whom
had cerebral infarctions confirmed on computed tomography.
Ninety-three of the 555 patients (16.8%) had evidence of
coronary artery disease, of whom 81 had features of myocardial
ischaemia, eight had pathological Q waves and four patients had
features of acute myocardial infarction. There has been long-
standing controversy regarding ECG diagnosis of myocardial
ischaemia in black Africans.
53-57
Ignoring ECG features of ‘ischaemia’ and ascribing such
changes to ‘normal variation’ poses the potential danger of
under-diagnosis or misdiagnosis of myocardial ischaemia in
black Africans. Rather, future work should attempt to unravel
the genetic mechanisms behind abnormal ECG patterns in black
Africans.
The combination of clinical assessment, chest radiograph,
resting electrocardiography, transthoracic echocardiography and
MUGA scanning showed features of CHD in 18 patients
(17.6%) in the MEDUNSA study. Scintigraphy with or without
dipyridamole infusion in 60 stroke patients in this study revealed
features of coronary heart disease in 45% of the patients.
Macroscopic and microscopic pathological examinations of the
Fig. 2. Cardiovascular disease risk factors in HIV-infected
patients in Botswana.
60
50
40
30
20
10
0
<30 30–34 35–39 40–44 45–49 50–54 55–59 60–64 65+
Number of affected HIV patients
Hypertension
Disglycaemia
Dyslipidaemia
Obesity
Smoking
Source: BOMAID/CCD database, 2008 (total of 501 HIV infected patients)
Age range (years)



















