CARDIOVASCULAR JOURNAL OF AFRICA • Volume 25, No 1, January/February 2014
16
AFRICA
study we aimed to examine atrial electromechanical coupling
and PD, reflecting inter-atrial conduction times in pregnant
subjects.
Methods
We consecutively studied 40 pregnant subjects. Eight were
excluded from the study, because of thyroid dysfunction in
three subjects, DM in three, unclearly identifiable P waves in
two, and bundle branch block in two. The study population
was composed of 30 pregnant subjects (mean age 28
±
4 years)
and 30 age-matched controls (mean age 28
±
3 years). All the
pregnant women were in the second trimester between 18 and
23 weeks. Physical examination, medical history of the patients
and blood biochemistry were evaluated in both groups to exclude
systemic diseases.
Subjects with coronary artery disease, heart failure, rheumatic
valve disease, primary cardiomyopathy, DM, hypertension,
thyroid dysfunction, any previous arrhythmia, anaemia,
electrolyte imbalance, chronic lung disease, and bundle branch
block and atrio-ventricular conduction abnormalities on ECG
were excluded from the study. Also, ECGs without clearly
identifiable P waves were excluded from the PD analysis using
standard 12-lead surface ECGs.
All of the patients were in sinus rhythm and none was taking
medications such as anti-arrhythmics, tricyclic antidepressants,
antihistamines and antipsychotics. All patients signed informed
consent form. The local ethics committee approved the study.
Two-dimensional, M-mode, pulsed and colour-flow
Doppler echocardiographic examinations of all subjects were
performed by the same examiner with a commercially available
machine (Vivid 7 pro, GE, Horten, Norway, 2–4 mHz phased
array transducer). During the echocardiography, a one-lead
electrocardiogram was recorded continuously.
M-mode measurements were performed according to the
criteria of the American Society of Echocardiography.
12
LA
diameter, and LV end-systolic and end-diastolic diameters
were measured. LV ejection fraction (EF) was estimated using
Simpson’s rule. LV mass was calculated with the Devereux
formula.
13
Conventional Doppler echocardiography was performed and
pulsed-wave mitral flow velocities were measured from the
apical four-chamber view by inserting a sample volume to the
mitral leaflet tips. Mitral early diastolic velocity (E, cm/s), late
diastolic velocity (A, cm/s), E/A ratio (E/A), E deceleration
time (DT, ms), and isovolumetric relaxation time (IVRT, ms)
were determined. Each representative value was obtained from
the average of three measurements. The operator was blinded to
the clinical details and results of the other investigations of each
pregnant subject and control.
Tissue Doppler imaging echocardiography was performed
with transducer frequencies of 3.5–4.0 MHz, adjusting the
spectral pulsed Doppler signal filters until a Nyquist limit of
15–20 cm/s was reached and using the minimal optimal gain.
The monitor sweep speed was set at 50–100 mm/s to optimise
the spectral display of myocardial velocities.
Myocardial peak systolic (Sm, cm/s), and early (Em, cm/s) and
late (Am, cm/s) diastolic velocities, Em/Am ratio, isovolumetric
contraction time (ICT, ms), isovolumetric relaxation time (IRT,
ms) and ejection time (ET, ms) were obtained by placing a tissue
Doppler sample volume in the basal segments of the anterior,
inferior, lateral, and septal wall.
14
The tricuspid annular motion
was recorded at the right ventricular (RV) free wall. Myocardial
performance index (MPI) was calculated using the (ICT + IRT)/
ET formula.
15
By calculating the arithmetical mean value of the
segmentary values, mean LV Sm, Em, mean Am, mean MPI, and
Em/Am values were obtained.
Tissue Doppler velocities therefore represent an average of
the basal segments of the anterior, inferior, lateral and septal
walls. Also, the E/Em ratio, an important non-invasive marker
of pulmonary capillary wedge pressure and LV filling pressure,
was calculated. Diastolic dysfunction was defined according to
the guidelines of the European Association of Echocardiography/
American Society of Echocardiography as the presence of septal
Em
<
8 cm/s, lateral Em
<
10 cm/s and LA volume
≥
34 ml/m
2
.
16
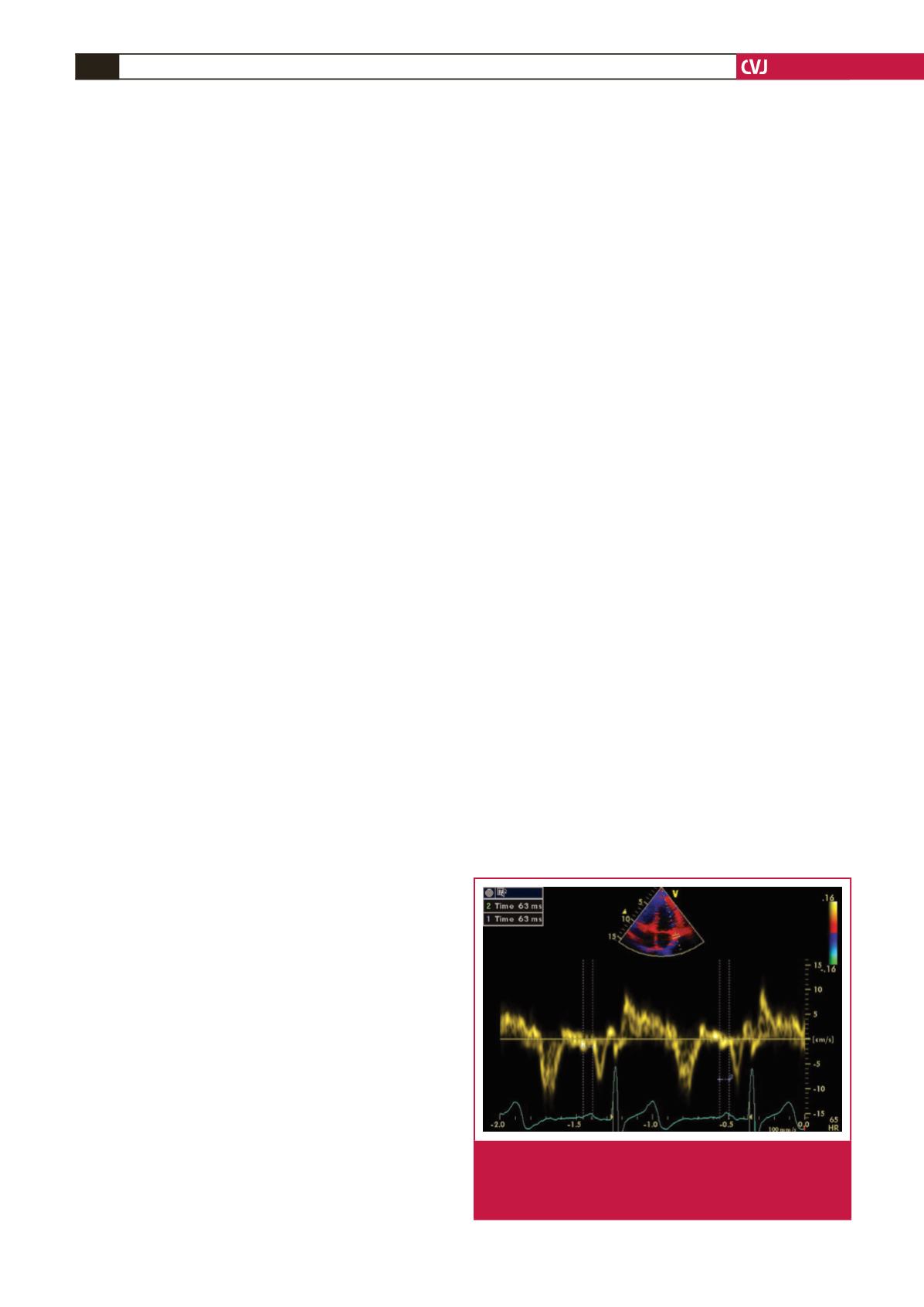
Atrial electromechanical coupling was determined as follows.
In an apical four-chamber view, the pulsed Doppler sample
volume was placed at the level of the LV lateral mitral annulus,
septal mitral annulus, and RV tricuspid annulus. The time
interval from the onset of the P wave on a surface ECG to the
beginning of the late diastolic wave (Am), which is termed
PA, was obtained from the lateral mitral annulus (PA lateral),
septal mitral annulus (PA septal), and RV tricuspid annulus (PA
tricuspid) (Fig. 1). The difference between PA lateral and PA
tricuspid (PA lateral – PA tricuspid) was defined as the inter-
atrial electromechanical coupling interval; PA septum and PA
tricuspid (PA septum – PA tricuspid) was defined as intra-atrial
electromechanical coupling interval; and the difference between
PA septal and PA lateral (PA septal – PA lateral ) was defined as
intra-left atrial electromechanical coupling interval.
18
P-wave dispersion was measured on 12-lead ECGs. All
standard 12-lead ECGs were obtained simultaneously using a
recorder (Hewlett Packard, Pagewriter) set at a 50-mm/s paper
speed and 1-mV/cm standardisation. ECG measurements were
evaluated on the same day, in a one-month period in our routine
practice. A single cardiologist, who was blinded to the clinical
status of the subjects, measured ECG intervals. To decrease the
error measurements, P-wave analysis was done with calipers and
magnifying glass.
Fig. 1.
Measurement of the time interval from the onset of
the P wave on a surface ECG to the beginning of the
A-wave (PA) interval using tissue Doppler echocardi-
ography.


