
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 4, July/August 2015
e10
AFRICA
Discussion
Cardiac involvement in lymphomas is a rare clinical presentation
with a dismal prognosis, occurring either as a primary cardiac
lymphoma,
1
or, more frequently, as secondary involvement
in the late evolution of aggressive lymphomas.
2
The clinical
presentation of cardiac involvement in lymphoma is variable.
Often, diagnosis is made by routine echocardiography. This is
regularly performed because evaluation of cardiac function is
important for assessment of the tolerability and side effects of
chemotherapy, especially in anthracycline-containing regimens,
which are known for their cardiotoxicity.
3,4
Even if the frequency of this type of tumour appears to be
increasing, real epidemiological figures are unknown, helped by
the fact that involvement of the heart is often asymptomatic.
Although rarely diagnosed during neoplastic clinical evolution,
cardiac metastases have been found in more than 10% of post
mortem examinations of patients succumbing to cancer,
5
more
frequently in melanoma, lung and breast cancer.
In lymphoma patients, post mortem figures for cardiac
involvement range from nine to 20%.
5,6
The diagnostic difficulty
in late evolution of non-Hodgkin lymphoma is associated with
the fact that heart failure may also be due to cumulative cardiac
toxicity of multiple lines of treatment and of the toxic cardiac
effects of anthracyclin-based chemotherapy regimens.
7,8
The
role of
18
F-FDG PET scans in detecting cardiac involvement
of lymphoma has been described in several case reports of
extralymphatic tumour involvement in lymphoma.
9,10
In our patient, cardiac involvement appeared as a late
evolution of an aggressive DLBCL. After four lines of chemo-
immunotherapy, including autologous stem cell transplantation,
the patient relapsed with mediastinal lymphadenopathy. Because
of the high cumulative dose of anthracyclines, well known for
their cardiotoxic effect, we chose to continue with a regimen
containing less cardiotoxic pegylated anthracycline, associated
with alkylating agent cyclophosphamide. After two cycles
the patient developed clinical signs of right cardiac failure.
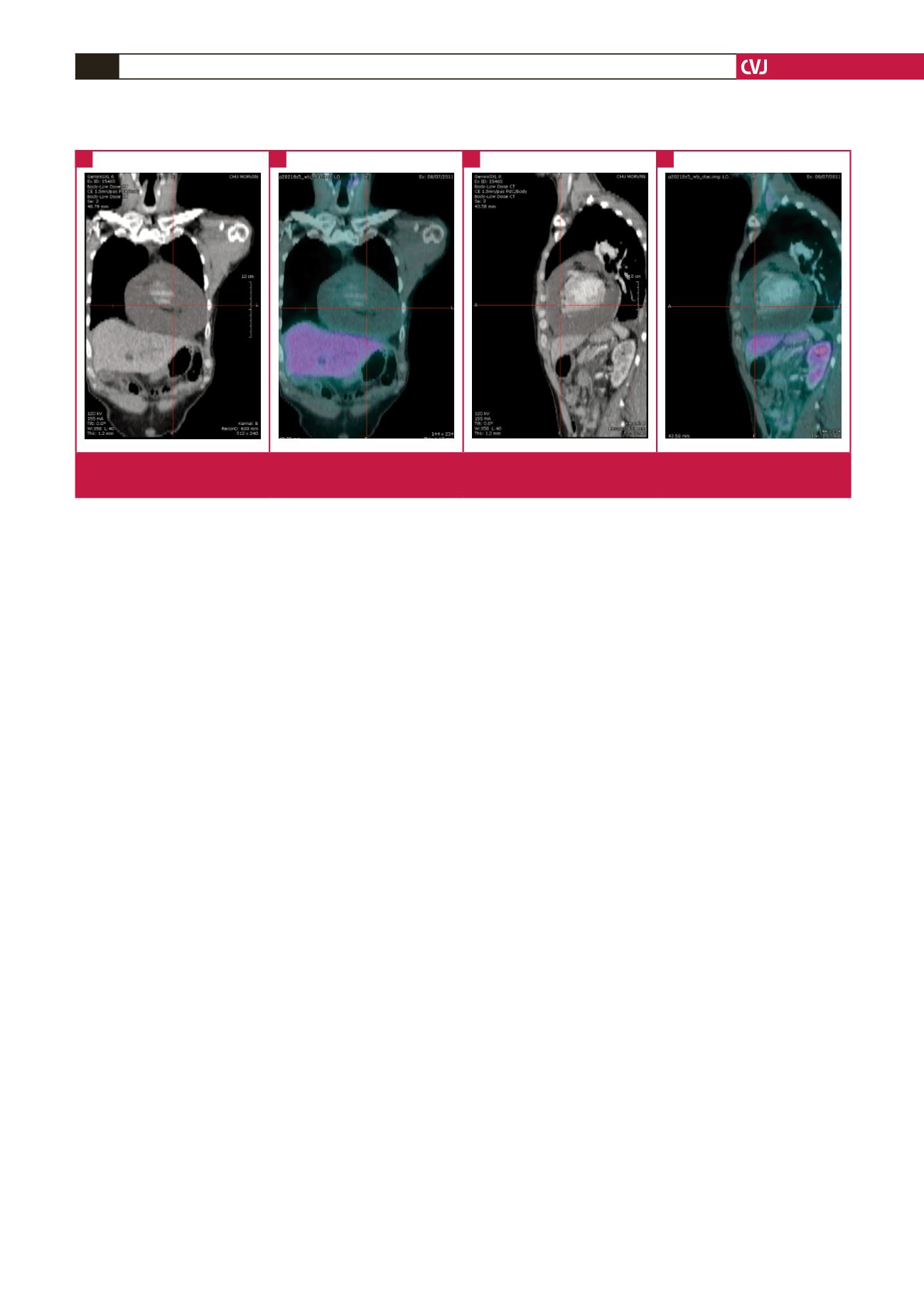
Examination by PET scan found the presence of a massive
pericardial effusion (Fig. 1), but discordantly, complete remission
of the initial localisations of the lymphoma.
Our first hypothesis on the cause of the effusion was
the cumulative toxic effect of chemotherapy, but cytology,
immunophenotyping and cytogenetic analysis of the liquid
obtained by puncture showed the presence of lymphoma.
Lymphoproliferative disease is regarded as systemic and
pericardial, i.e. extralymphatic involvement is a sign of the
highest degree of dissemination in lymphoma staging, warranting
systemic therapy after removal of the pericardial effusion fluid,
rather than performing pericardiectomy or pericardial sclerosis.
Conclusion
We suggest that, if signs or symptoms of cardiac failure develop
during or after chemotherapy for lymphoma, the hypothesis of
cardiac involvement of lymphoma should be considered. The
diagnosis of this usually late complication requires cytological
confirmation.
This work was supported by grant 1494 /2014 from the University of
Medicine and Pharmacy Cluj (to M Zdrenghea) and the European Social
Fund, Human Resources Development Operational Programme 2007-2013,
project POSDRU/159/1.5/S/138776 (to C Bagacean and M Zdrenghea). The
sponsors had no involvement in the collection, analysis and interpretation of
data, the writing of the manuscript, and the decision to submit the manuscript
for publication.
References
1.
Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary
cardiac lymphoma in immunocompetent patients: diagnostic and thera-
peutic management.
Cancer
1997;
80
: 1497–1506.
2.
Mikdame M, Ennibi K, Bahrouch L, Benyass A, Dreyfus F, Toloune
F. [Cardiac localization of non Hodgkin’s lymphoma: a study on four
cases].
Rev Med Interne
2003;
24
: 459–463.
3.
Sarjeant JM, Butany J, Cusimano RJ. Cancer of the heart: epidemiology
and management of primary tumors and metastases.
Am J Cardiovasc
Drugs
2003;
3
: 407–421.
4.
Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and
pathologic characteristics of primary cardiac tumors: a study of 149
Fig. 1.
Coronal and sagittal CT and fused PET-CT reformatted images demonstrate large pericardial effusion (arrow). No abnormal
FDG uptake is noted.
A
C
B
D

















