
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 27, No 4, July/August 2016
210
AFRICA
its long-term outcomes. The aim of our study was to describe
this small, new-generation pump and the first implantation
experience from Istanbul, Turkey.
Methods
From December 2011 to December 2013, Heart Assist 5 LVADs
were implanted in nine patients at our hospital. Eight patients
had ischaemic cardiomyopathy and one had adriamycin-induced
cardiomyopathy. The mean age of the patients was 53
±
13
(34–64) years (Table 1).
All patients’ pre-operative data (catheterisation,
echocardiography, laboratory and infection parameters) and
haemodynamic status had been assessed at the hospital’s medical
review board meeting to evaluate their selection for LVAD
implantation. All patients had signed informed consent and the
study protocol was approved by the institutional review board.
In December 2011, 55 serial two-dimensional transthoracic
echocardiographic (TTE) examinations had been performed
on these patients with the Vivid 3 (General Electric, Fairfield,
Connecticut), and 16 serial three-dimensional TTE examinations
had been done with the Philips IE33 xMATRIX (Royal Philips
Electronics, Amsterdam, Netherlands). Nine transoesophageal
echocardiograms (TEE) were done in the operating room
under general anaesthesia during the LVAD implantation to
evaluate inflow cannula and septum position, and to monitor
the de-airing process while weaning from cardiopulmonary
bypass. The specific protocol used for these echocardiographic
studies included standard TTE parasternal, apical, subcostal and
suprasternal notch views.
1-13
All TTEs and TEEs were performed by certified cardiologists
in different clinical settings. In hospital during the pre-implant
period, they evaluated the intracardiac structure [patent foramen
ovale (PFO), atrial septal defect (ASD)], chamber dimensions,
left ventricular (LV) and right ventricular (RV) function, valvular
structure and function, pericardial disease, volume status and
abnormalities of the aorta. In hospital during the post-implant
LVAD period, the aim was to visualise the septum and inflow
cannula position, the intracardiac volume, outflow conduit,
decompression of the left ventricle with rpm speed change and
its effects on the aortic valve opening time. Doppler interrogation
of the inlet cannula and outflow conduit flows were performed
as described in the literature.
10,12,13
Follow-up TTEs were done for
clinical indications, including LV and RV ejection fraction (EF),
RV fractional area contraction (RVFAC), unexplained change in
haemodynamic status including minor dehydration, ventricular
tachycardia attacks, suspicion of device malfunction, excess
current alarms, and optimisation of LVAD speed.
Echo-specific parameters and differences in image quality or
artifact generation were not evaluated in our study. During all
echocardiographic studies, the transplant surgeons were at the
bedside or in the out-patient clinic, in communication with the heart
failure cardiologist setting up the pump parameters, optimising the
LVAD speed, and observing the aortic valve opening times and
septum inflow cannula position to prevent the suction cascade.
Images from 55 TTEs were retrospectively analysed by the
heart failure cardiologist who had mainly performed all these
echo studies. The inlet cannula/outflow conduit velocities were
measured in m/s by spectral Doppler. The pulsality index of the
pump, LV end-diastolic dimension (LVEDD), LV end-systolic
dimension (LVESD), interventricular septum (IVS), posterior
wall thickness, RVFAC, and tricuspid annular plane systolic
excursion (TAPSE) were measured, and the functioning of the
valves was routinely evaluated pre-implant and at the second
week, and first, third, sixth, ninth and 12th months post-implant.
Statistical analysis
All echocardiographic data were collected in parallel with the
patients’ laboratory parameters (biochemical, blood count,
coagulation profile) in an electronic database, and descriptive
statistics were calculated using Microsoft Excel (Microsoft Corp,
Redmond, Washington). Continuous variables were expressed as
the mean value
±
standard deviation.
Results
As our Heart Assist 5 LVAD patient data were limited, we did
not compare the patients’ outcome parameters pre- versus post-
implant, knowing that with this small group, all analyses would
be statistically non-significant. We therefore analysed these
patients’ data as descriptive statistics.
The titanium impeller of Heart Assist 5 LVAD is small, light-
weight and located pericardially. The titanium housing enables
direct visualisation of the impeller. The outflow and inflow
cannulas were visualised in all studies. The inflow cannula and
septum (left, right, neutral) position, outflow graft anastomosis
to the aorta, aortic valve cusp status, and aortic valve opening
times with speed changes were evaluated and visualised in all 55
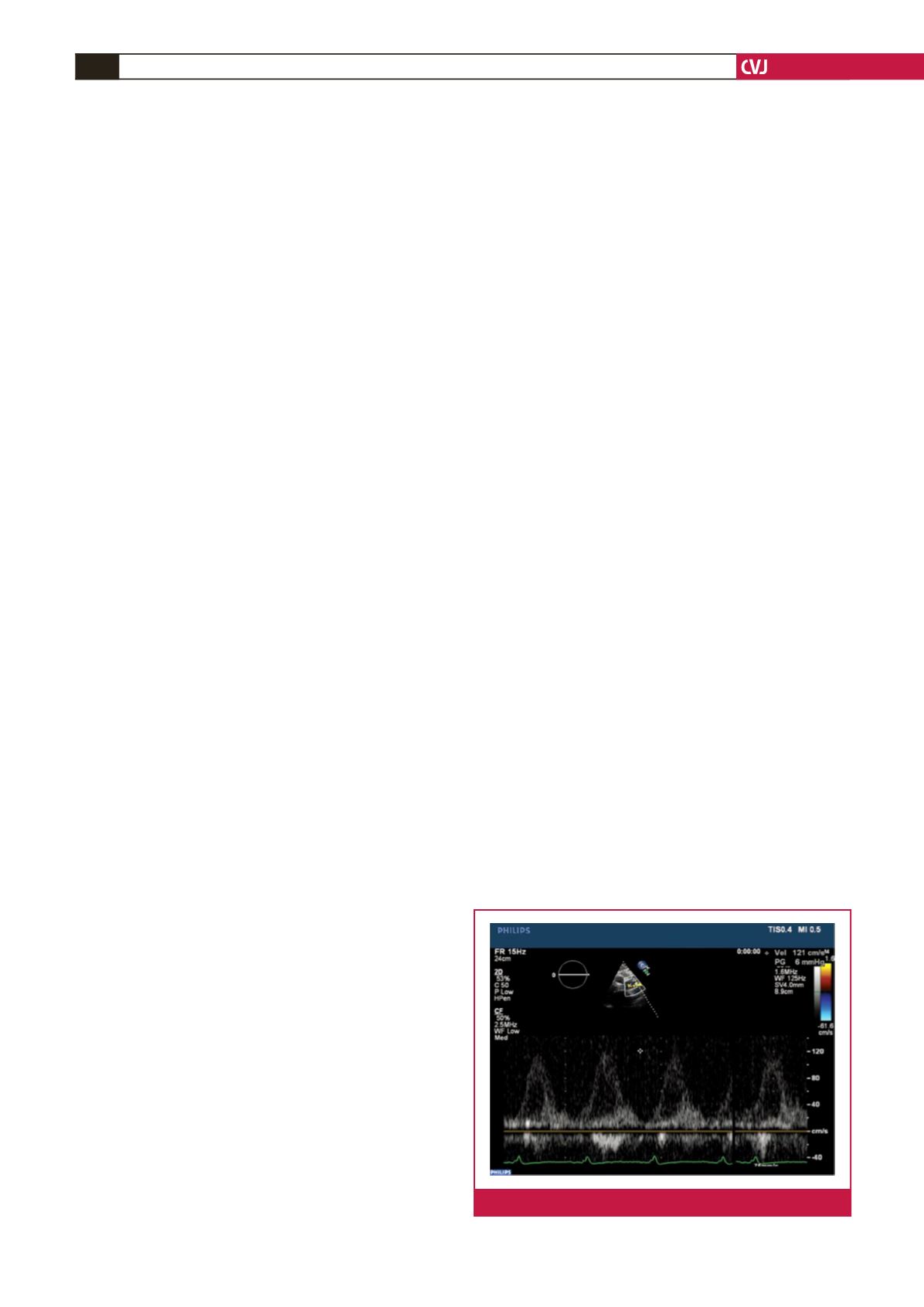
echocardiographic studies (Figs 4–6).
Pre-implant echocardiographic data of the six patients: mean
EF was 23
±
5 (18–28)%, mean LVEDD was 6.9
±
0.6 (6.3–7.7)
cm, mean LVESD was 5.8
±
0.5 (5.1–6.4) cm, mean IVS was 0.9
±
0.1 (0.9–1.1) cm, mean RVFAC was 43
±
9 (35–55)%, and mean
TAPSE was 17
±
4 (13–23) mm.
The 12-month post-implant echocardiographic data of the
six patients were: mean EF was 19
±
6 (10–25)%, mean LVEDD
was 6.4
±
0.4 (6.1–7.0) cm, mean LVESD was 5.6
±
0.3 (5.2–6.0)
cm, mean RVFAC was 35
±
11(21–43), mean TAPSE was 13
±
2
(11–16) mm, mean rpmwas 9 800
±
600 (9 500–10 400) rpm, mean
pulsality index (PI) was 2.79
±
1.7 (1.9–4.9) m/s, outflow cannula
Fig. 4.
Outflow cannula velocity measurement with Doppler.

















