
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 2, March/April 2017
AFRICA
135
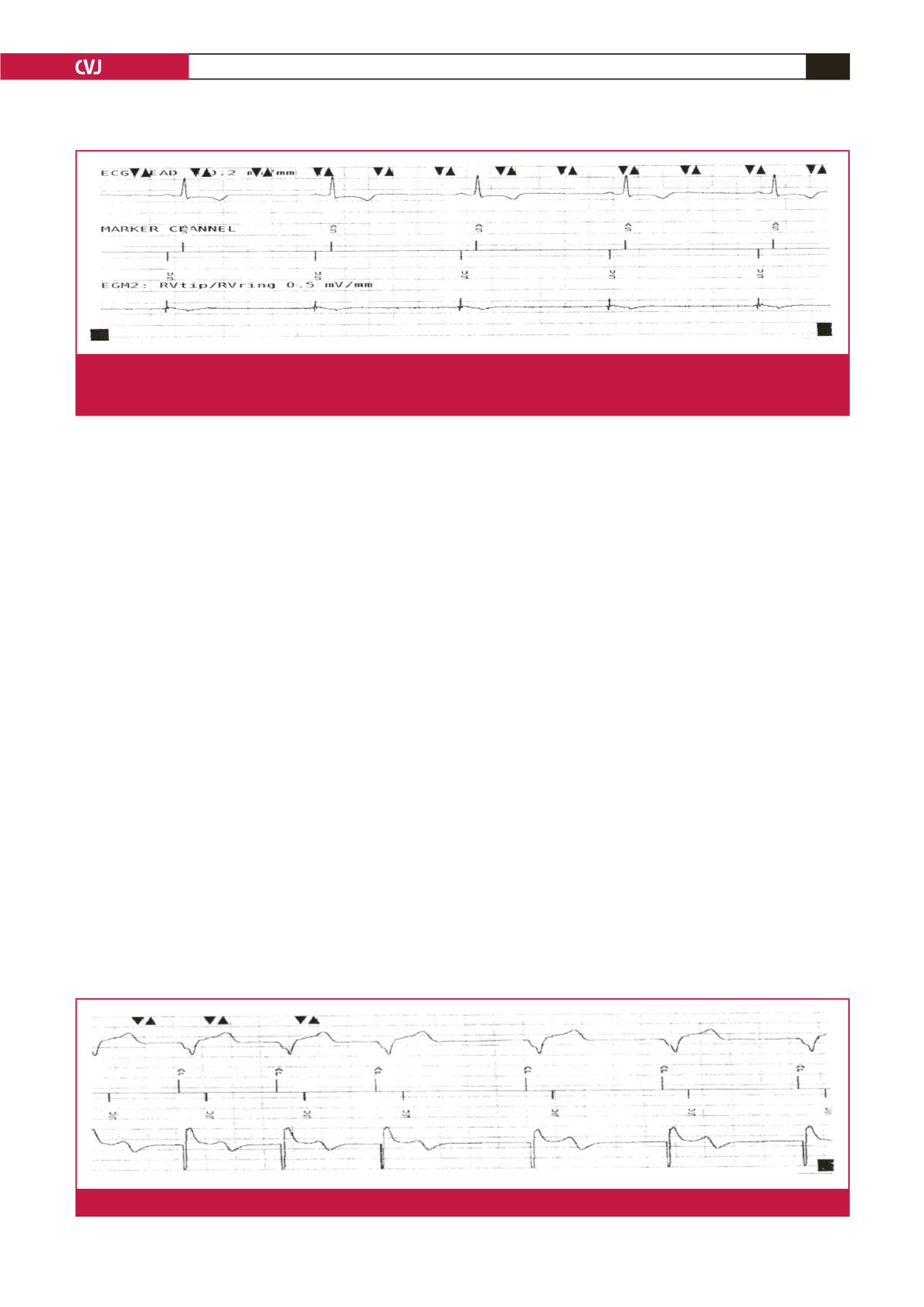
(ECG) showed paced QRS with P wave at the end of the QRS
complex, indicative of atrioventricular dyssynchrony (Fig. 1).
These clinical findings, together with the ECG, raised the
suspicion of pacemaker syndrome.
Pacemaker interrogation showed that his pacemaker was
programmed to AAIR–DDDR mode with a base rate of 70
bpm; the battery power was fine. The ECG showed typical right
ventricular pacing compatible with VVIR mode despite AAIR–
DDDR programming. Moreover, an atrial electrogram (EGM)
showed ventricular pacing and a ventricular EGM showed
sensed atrial depolarisation (Fig. 2). These findings were highly
suggestive of atrial/ventricular lead switch at the pacemaker
header. The underlying rhythm was sinus with an intrinsic rate
of 45 bpm. Chest X-ray and fluoroscopy showed that the atrial
and ventricular leads were situated in the correct positions in the
respective chambers.
The patient subsequently underwent a corrective procedure
(lead repositioning) without temporary pacing cover. During
the procedure, it was confirmed that the leads were switched,
with the ventricular lead connected to the atrial port, and the
atrial lead connected to the ventricular port. Both the atrial and
ventricular leads were disconnected and tested, after which they
were reconnected to the appropriate ports at the pacemaker
header. Atrial pace and ventricular sense were achieved through
the AAIR–DDDR pacing mode (Fig. 3).
It was noteworthy that prior to correction, the blood pressure
was 108/62 mmHg, with a HR of 45 bpm. Post correction, the
blood pressure immediately rose to 141/77 mmHg, with a HR of
72 bpm (AP–VS).
An echocardiogram done later the same day showed that
the LVEF had dropped from 74 to 49% post pacemaker
insertion. This high LVEF was thought to have been due to
right ventricular-only pacing-induced left ventricular systolic
dysfunction.
The differential diagnosis for pacemaker syndrome includes:
acute coronary syndromes, hyperthyroidism, hypothyroidism,
pacemaker failure, pacemaker-mediated tachycardia and
cardiogenic pulmonary oedema, among others.
5
In our case, the
rise in systolic blood pressure of > 20 mmHg post correction of
the leads confirmed the diagnosis of pacemaker syndrome.
The above differential diagnoses were ruled out as follows:
the cardiac biomarkers were negative with no ST changes on
the ECG, which ruled out an acute coronary syndrome. The
patient was biochemically euthyroid with no clinical features
of hyper- or hypothyroidism. The pacemaker was functional,
ruling out the possibility of pacemaker failure. The resting heart
rate was less than 100 bpm and this excluded the possibility
of pacemaker syndrome in this case being due to pacemaker-
medicated tachycardia. The patient was clinically not in left
ventricular failure, with clear lung fields on chest radiography
and on auscultation, and therefore cardiogenic pulmonary
oedema was an unlikely cause of his symptoms. Pulmonary
embolism was also ruled out based on a negative D-dimer
laboratory result.
Discussion
Pacemaker syndrome is defined as intolerance to ventricular-
based (VVIR) pacing due to loss of atrioventricular (AV)
synchrony.
6
It is an iatrogenic disorder that results from the
Fig. 2.
Electrogram showing typical right ventricular pacing, compatible with VVI mode despite AAIR–DDDR programming at the
pacemaker. This shows ventricular pacing when in fact pacing is from the right atrial lead. There is ‘atrial sensing’ from the
ventricular lead.
Fig. 3.
Twelve-lead ECG post lead repositioning showing proper atrial and ventricular pacing in AAIR–DDDR pacing mode.

















