
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 28, No 6, November/December 2017
AFRICA
347
Methods
Ethical approval for the study was obtained from the Human
Research Ethics Committee of the Faculty of Medicine and
Health Sciences of the University of Stellenbosch (U15/09/002).
We conducted a retrospective review of adult patients (18
years or older) admitted to Tygerberg hospital (TBH), Cape
Town, with warfarin toxicity during a one-year period from June
2014 to June 2015. Only patients known to be on established
warfarin therapy were eligible for inclusion. Patients who
were initiated on warfarin therapy during admission were
excluded. Patients admitted more than once during the study
period were recorded separately for each admission. We used
National Health Laboratory Service (NHLS) records to identify
patients with raised INRs and reviewed clinical notes, laboratory
investigations and prescription data.
We collected demographic information, admission and
discharge dates, INR measurements, the presence or absence
of bleeding, sites and complications of bleeding, management,
presumed cause of warfarin toxicity as recorded in the clinical
notes, and whether it was addressed prior to discharge, as well
as concomitant medicine use at time of admission. The cause of
warfarin toxicity was recorded as not identified if a cause was
not recorded in the clinical records. In the presence of bleeding,
we classified it as major or non-major bleeding. Major bleeding
was regarded as life- or limb-threatening bleeding, whereas all
other cases where regarded as non-major bleeding.
We included patients presenting with warfarin toxicity, as
defined by an admission INR value greater than 5. Patients
included required at least one additional in-patient INR
measurement to capture only in-patients. We excluded patients
with an elevated INR who were not using warfarin and presented
with elevated INRs due to other pathology such as liver
impairment and sepsis. Patients with one elevated INR reading
but who died prior to an additional INR measurement being
done were not eligible for inclusion.
We calculated the warfarin toxicity treatment cost using the
procurement cost of blood and blood products from the Western
Cape Blood Transfusion Service, procurement cost of medicines
from the TBH pharmacy, and cost to the hospital to admit
a patient in a general ward in TBH using 2015 financial year
costing. The general ward admission cost included personnel
cost, clinical consumables, maintenance and engineering,
equipment, services and overhead costs.
We used DRUG-REAX
®
Interactive Drug Interactions
database (Truven Health Analytics Inc, Micromedex
®
Healthcare
Series)
8
to identify possible drug–drug interactions (DDI) between
warfarin and drugs used by patients at the time of admission.
Statistical analysis
No sample size was calculated and all patients identified during
the study period were included. Data were entered into Microsoft
Excel
®
and analysed using Stata version 11.0 (StataCorp, College
Station, TX, USA). We assessed the normality of the data
visually and using the Shapiro–Wilk test. Normally distributed
data are described using the mean and standard deviation
(SD) while non-normally distributed data are described using
median and interquartile ranges (IQR). We explored statistical
significance using appropriate tests for categorical, normal
numerical and non-normal numerical data.
Results
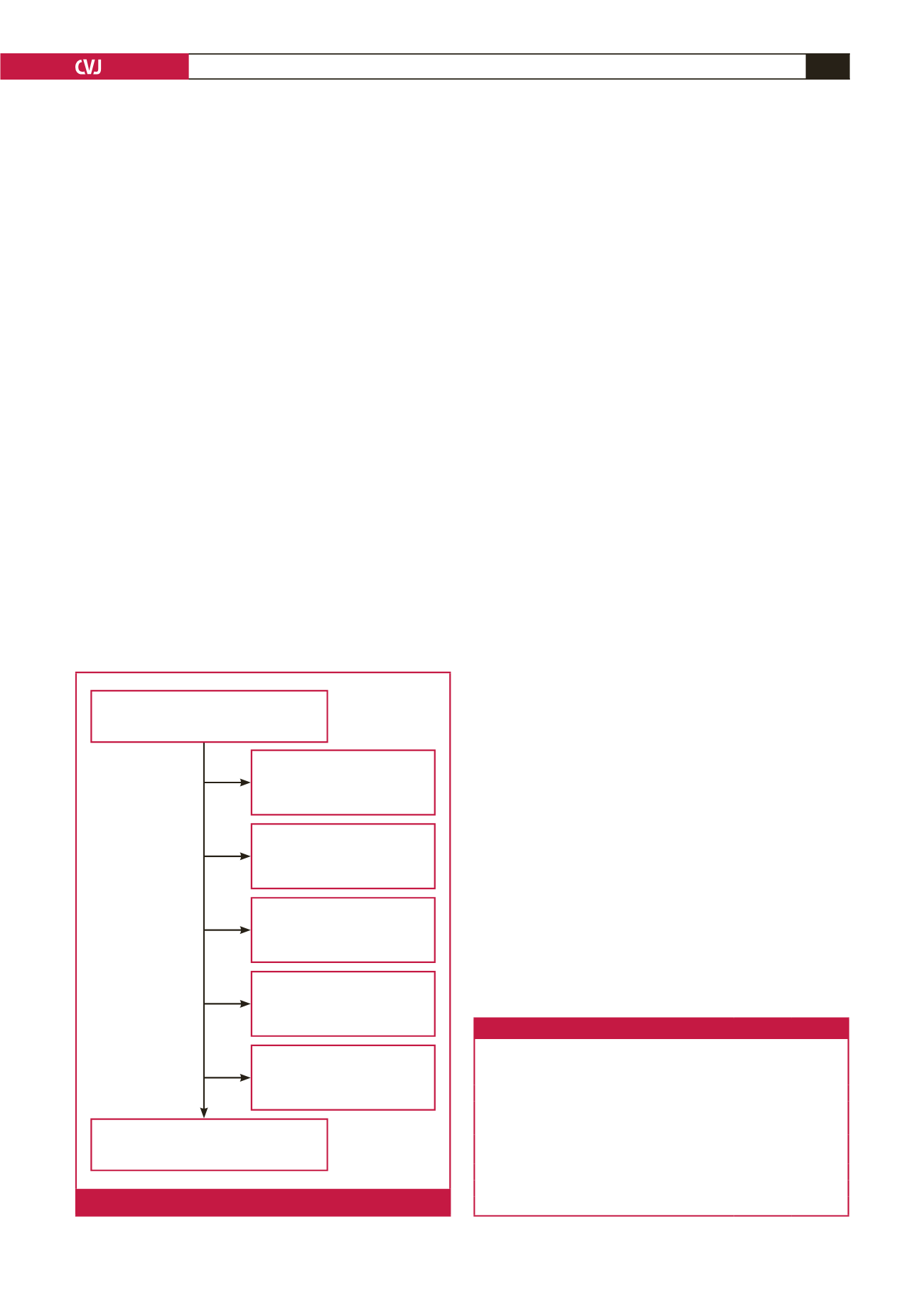
We identified 474 raised INR measurements (467 patients), of
which 126 (122 patients) met our inclusion criteria for warfarin-
toxicity admissions (Fig. 1). Four patients presented with two
admissions for warfarin toxicity during the study period and
each admission was recorded. For clarity we will refer to the 126
warfarin-toxicity admissions as patients.
Sixty per cent (76/126) of patients were female and 40%
(50/126) were male, with a median (IQR) age of 61 (48–70)
years. Fifteen per cent (19/126) of patients died before discharge,
although we could not attribute with certainty cause of death to
warfarin toxicity. Patients were admitted for a median (IQR) of
eight (5–16) days. The most common indications for the usage
Table 1. Indications for warfarin therapy
Indication
Number
of patients Percentage
AF
48
38
Heart valve replacements
24
19
DVT
21
17
Other
21
17
Multiple indications (including, but not limited to AF,
heart valve replacements and DVT)
9
7
Unknown
3
2
Total
126
AF
=
atrial fibrillation, DVT
=
deep-vein thrombosis.
Excluded due to no records
being available (
n
= 55)
Raised INR measurements identified
(
n
= 474)
Excluded due to not being
admitted (
n
= 24)
Excluded due to only one
INR measured (
n
= 46)
Excluded as not on warfarin
at admission (
n
= 187)
Excluded due to not being
admitted (
n
= 24)
Patients eligible for inclusion
(n = 126)
Fig. 1.
Study sample selection.

















