
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 6, November/December 2018
AFRICA
379
Japan, in contrast to the United States, Germany and India, who
mainly use the femoral route.
2
Moreover, there is an incomplete
evidence base for overall superiority of the radial approach over
the femoral approach.
Recently, two randomised, controlled trials were published
regarding clinical outcomes of TRI in patients with ACS.
5,7
The
RIVAL trial showed the clear benefit of TRI in terms of primary
outcome (a composite of death, MI, stroke and major bleeding)
(HR: 0.60, 95% CI: 0.38–0.94;
p
=
0.026) and mortality (HR:
0.39, 95% CI: 0.20–0.76;
p
=
0.006) at 30 days in patients with
STEMI.
5
Also in the RIFLE-STEACS study, TRI was associated
with significant clinical benefits in the primary outcome (a
composite of CD, stroke, MI, TLR and bleeding) (13.6 vs 21.0%,
p
=
0.003) and cardiac mortality rate (5.2 vs 9.2%,
p
=
0.020) at 30
days in patients with STEMI, compared with TFI.
7
Consistent
with these results, Karrowni
et al
. concluded from their meta-
analysis, including 12 randomised trials, that TRI was associated
with favourable outcomes in STEMI patients and should be the
preferred approach for experienced radial operators.
14
However, in contrast to STEMI, the clinical benefits of TRI
in NSTE-ACS have not been fully demonstrated. In subgroup
analysis of the RIVAL trial, TRI did not show any clinical
benefits in the primary outcome (HR: 1.11, 95% CI: 0.83–1.48;
p
=
0.49) and mortality rate (HR: 1.66, 95% CI: 0.94–2.92;
p
=
0.082) at 30 days.
5
Post hoc
analysis of the EARLY-ACS trial
revealed no significant differences in 30-day death/MI (12.6
vs 11.2%,
p
=
0.162) or 30-day death (1.8 vs 2.3%,
p
=
0.257)
between the TRI and TFI groups.
8
In our study, the rates of 30-day MACE and CD were no
different between the TRI and TFI groups (0.8 vs 0.4%,
p
=
0.545; 0.5 vs 0.0%,
p
=
0.283, respectively), nor were the rates
of one-year MACE and CD. Therefore our data corroborate
previous findings and extend the observations to one year.
Regarding bleeding complications, the subgroup analysis of
the PRESTO-ACS trial revealed that TRI was associated with a
significant decrease in TIMI bleeding (0.7 vs 2.7%,
p
=
0.03) at
one year in patients with NSTE-ACS.
9
Also, subgroup analysis of
the RIVAL trial showed lower rate of ACUITY-defined bleeding
with TRI in the NSTE-ACS cohort.
5
However, the results in
the previous two studies were derived from subgroup analysis,
which had the potential for false-positive errors.
15
Furthermore,
the former study was conducted without any sensitivity analysis
despite uneven distribution of baseline clinical, procedural and
pharmacological characteristics between the TRI and TFI groups.
9
Corroborating previous findings, our analysis was in line with
earlier results, using different bleeding criteria (BARC criteria)
in a significantly larger TRI cohort, although bleeding is an
outcome that is definition dependent.
16
Therefore, our study
strongly supports the benefit of TRI with regard to bleeding
complications in intervention for NSTE-ACS and may be more
reflective of real-world clinical practice.
For the paradigm shift from ‘femoral access first’ to ‘radial
access first’ in the middle of the debate on the preferred vascular
access site in PCI, there should be enough clinical evidence
supporting the efficacy and safety of TRI to TFI. However, there
is an incomplete evidence base for TRI, especially in patients
with NSTE-ACS.
In this study, we clearly demonstrated that TRI shows
comparable one-year clinical outcomes and lower bleeding
complications compared to TFI. In addition, for NSTE-ACS
patients, more will be on the more potent antiplatelet agents
such as prasugrel and ticagrelor, which have higher bleeding risks
than clopidogrel.
17,18
Therefore, TRI might become the vascular
access site of choice and the best option to decrease bleeding
complications, with favourable clinical outcomes in NSTE-ACS
intervention.
Study limitations
This study has several limitations. First, there may have been an
allocation bias based on uneven distribution of risk factors and
clinical and anatomical conditions of the patients, since this was a
non-randomised, observational study and the selection of vascular
access site was left to the operator’s discretion. To overcome this,
we used robust statistical methods, including propensity-score
matching and multivariate Cox proportional hazards regression.
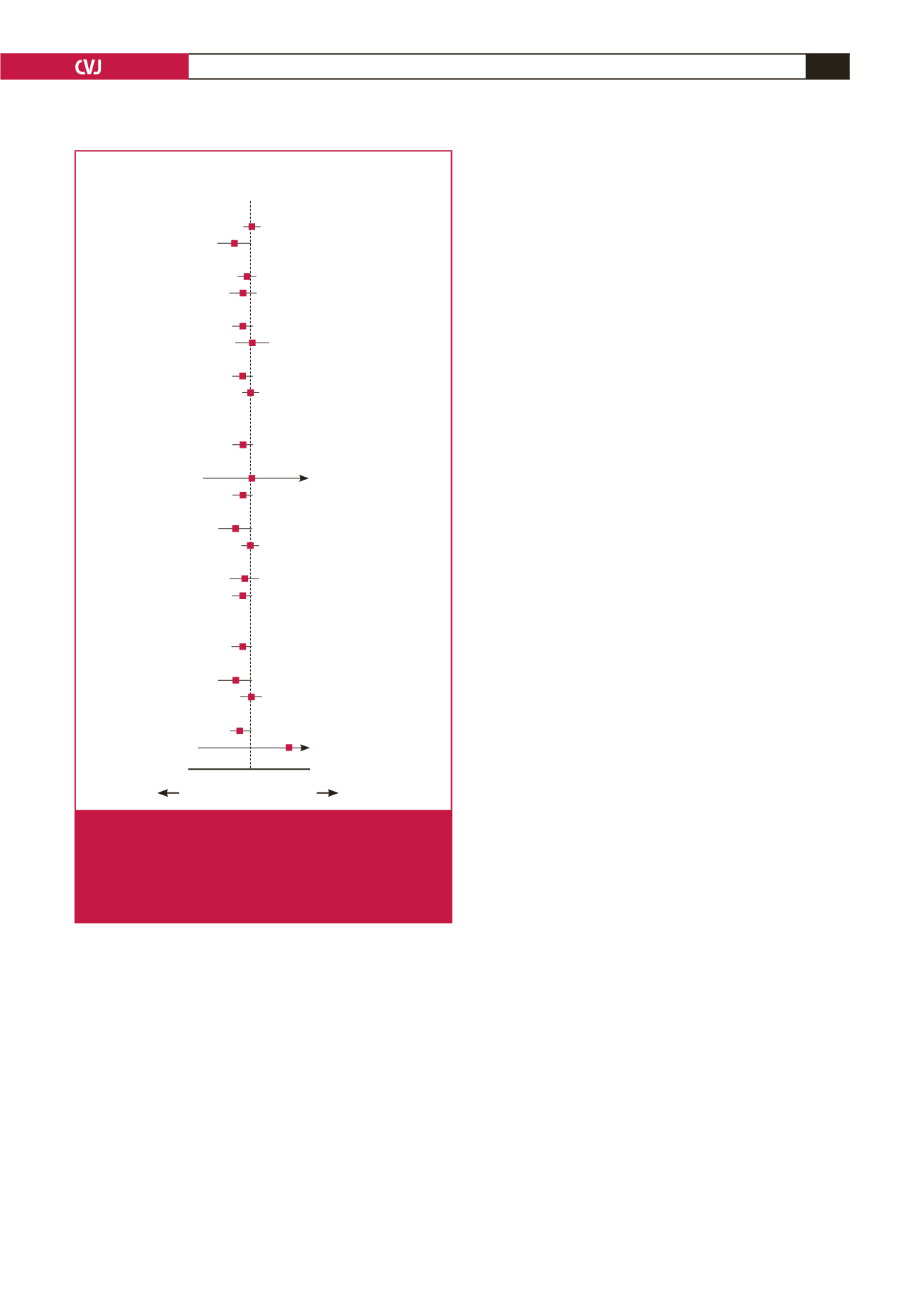
Variables
HR (95% CI)
Inter-
action
p
-value
Age
0.02 0.1 1.0 10 50
0.136
≥
65 years (
n
=
483)
1.109 (0.440–2.795)
≤
65 years (
n
=
382)
0.384 (0.135–1.094)
Gender
0.511
Men (
n
=
566)
0.813 (0.370–1.786)
Women (
n
=
299)
0.494 (0.133–1.840)
Hypertension
0.142
Yes (
n
=
546)
0.492 (0.218–1.107)
No (
n
=
319)
1.535 (0.428–5.502)
Diabetes mellitus
0.408
Yes (
n
=
290)
0.564 (0.227–1.402)
No (
n
=
575)
1.002 (0.361–2.784)
Dyslipidaemia
0.999
Yes (
n
=
126)
NA
No (
n
=
739)
0.722 (0.370–1.412)
Chronic kidney disease
0.652
Yes (
n
=
8)
1.414 (0.085–23.573)
No (
n
=
857)
0.738 (0.369–1.477)
Current smoker
0.208
Yes (
n
=
248)
0.402 (0.135–1.197)
No (
n
=
617)
0.996 (0.416–2.384)
History of IHD
0.876
Yes (
n
=
252)
0.774 (0.227–2.646)
No (
n
=
613)
0.693 (0.311–1.542)
History of PAD
>
0.999
Yes (
n
=
16)
NA
No (
n
=
849)
0.710 (0.363–1.389)
NSTEMI
0.182
Yes (
n
=
299)
0.417 (0.146–1.189)
No (
n
=
566)
1.062 (0.422–2.676)
Multi-vessel disease
0.898
Yes (
n
=
507)
0.525 (0.257–1.071)
No (
n
=
358)
32.597
(0.026–40335.924)
TRI better
TFI better
Fig. 3.
Subgroup analysis for major adverse cardiovascular
events in the propensity-score matched population
at one year. IHD
=
ischaemic heart disease; PAD
=
peripheral artery disease; NSTEMI
=
non-ST-segment
elevation myocardial infarction; TRI
=
transradial inter-
vention; TFI
=
transfemoral intervention.

















