
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 2, March/April 2019
e8
AFRICA
Discussion
Growth hormone is a peptide hormone released from the
anterior pituitary somatotroph cells and is involved in growth,
cell division and regeneration, regulation of metabolism, the
immune and cardiovascular systems, and brain function. The
effects of growth hormone are directly mediated via GHR and
indirectly via IGF1.
1,2
Laron syndrome, also known as growth hormone-insensitivity
syndrome, is an autosomal recessive disorder that causes
insensitivity to growth hormone and is characterised by short
stature. It is associated with inadequate generation of IGF1 in
response to growth hormone, due to dysfunction of the GHR.
Laron syndrome is caused by diverse GHR gene mutations,
including deletions, RNA processing defects, translational stop
codons and missense codons. All the identified mutations involve
the extracellular domain of the receptor and most are unique to
families or geographic areas.
3,4,7,8
Cardiac abnormalities are rare in patients with Laron
syndrome, but cardiac hypertrophy may be seen after IGF1
therapy.
4,5
In this report, we present a patient with Laron
syndrome related to homozygous GHR c.784
>
C mutation,
with hypoplastic pulmonary arteries and severe peripheral-
type pulmonary stenosis. The second angiography showed that
the pulmonary arteries had improved slightly. Our surgeons
planned patch augmentation for the hyoplastic pulmonary
arteries after the second cardiac catheterisation. We also detected
a novel mutation in our patient. To the best of our knowledge,
pulmonary hypoplasia and pulmonary stenosis have not been
reported before in a patient with Laron syndrome.
Conclusion
Cardiac abnormalities such as patent ductus arteriosus or
peripheral vascular disease are rare in patients with Laron
syndrome, but cardiac hypertrophy has been observed after
IGF1 therapy. Here we report on a 10-year-and-5-month-old girl
with severe pulmonary artery hypoplasia and Laron syndrome
related to homozygous GHR c.784
>
C mutation.
References
1.
Laron Z. Lessons from 50 years of study of Laron syndrome.
Endocr
Pract
2015;
21
(12): 1395–1402.
2.
Janecka A, Kołodziej-Rzepa M, Biesaga B. Clinical and molecular
features of Laron syndrome, a genetic disorder protecting from cancer.
In Vivo
2016;
30
(4): 375–381.
3.
Kurtoglu S, Hatipo
ğ
lu N. Growth hormone insensitivity: diagnostic and
therapeutic approaches.
J Endocrinol Invest
2016;
39
(1): 19–28.
4.
Erol N, Yıldız M, Güven A, Yıldırım A. Cardiac examination in chil-
dren with Laron syndrome undergoing mecasermin therapy.
J Pediatr
Endocrinol Metab
2018;
312
(6): 675–677.
5.
Feinberg MS, Scheinowitz M, Laron Z. Echocardiographic dimensions
and functions in adults with primary growth hormone resistance (Laron
syndrome).
Am J Cardiol
2000;
85
(2): 209–213.
6.
Akıncı A, Rosenfeld RG, Hwa V. A novel exonic GHR splicing muta-
tion (c. 784 G
>
C) in a patient with classical growth hormone insensivity
syndrome.
Horm Res Paediatr
2013;
79
: 32–38.
7.
Lin S, Li C, Li C, Zhang X.Growth hormone receptor muations related
to individual dwarfism.
Int J Mol Sci
2018;
19
(5): 1433.
8.
Laron Z, Kauli R, Lapkina L, Werner H. IGF-1 deficiency, longevity
and cancer protection of patients with Laron syndrome.
Mutat Res Rev
Mutat Res
2017;
772
: 123–133.
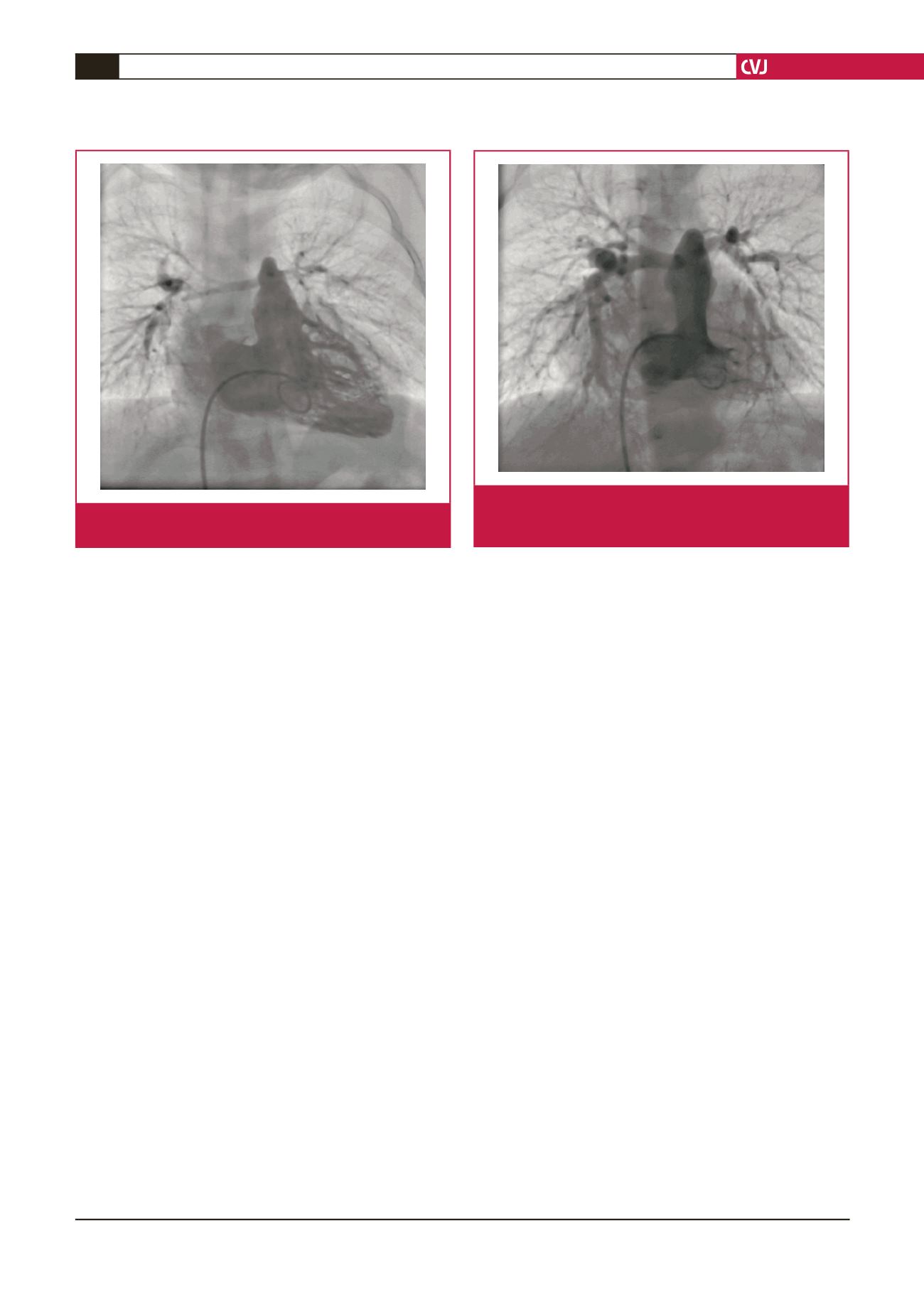
Fig. 2.
Second right ventricular angiography shows hypo-
plasia of the pulmonary arteries. The size of the
pulmonary arteries had improved slightly.
Fig. 1.
Right ventricular angiography shows hypoplasia of the
pulmonary arteries.

















