
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 4, July/August 2019
AFRICA
223
(CAD).
18-20
In those studies, perindopril improved the parameters
of diastolic function in patients with HT and CAD but did not
affect diastolic function in diabetic patients.
The effects of perindopril on the echocardiographicparameters
of diastolic and systolic function have not been previously
investigated in patients with DHF. Given the aforementioned
studies, we aimed to investigate the effects of perindopril on
the parameters of diastolic left atrial function and longitudinal
myocardial function with TDE in patients with DHF. We also
investigated whether perindopril treatment changed the NYHA
functional class and serum NT-proBNP levels in this study
population.
Methods
We enrolled 108 patients with DHF, aged
≥
50 years, who
presented to Izmir Ataturk Education and Research Hospital
(IAERH) cardiology out-patient clinics with HF symptoms
and were found to have an ejection fraction (EF) of
≥
50%
on transthoracic echocardiography (TTE) accompanied by
an evidence of DD on TDE. The HF diagnosis was based on
Framingham heart failure criteria.
Exclusion criteria included the following: EF
<
50%, patient age
<
50 years, severe valvular disease on echocardiography, a history
of acute coronary syndrome, atrial fibrillation, cardiomyopathy
or pericardial disease, anaemia (serum haemoglobin levels
<
10 g/
dl), hyperthyroidism, hypothyroidism, renal insufficiency (serum
creatinine
>
2 mg/dl or dialysis), serum potassium level
>
5.5
mEq/l, moderate to severe pulmonary hypertension (sPAP
>
50
mmHg), and/or intolerance to angiotensin converting enzyme
inhibitors (ACEIs) or angiotensin receptor blockers (ARBs),
bilateral renal artery stenosis, or any kind of malignancy.
Patients with decompensated HF were also excluded.
The patients were randomised into two groups using a basic
randomisation method. The first group (perindopril group) was
started on oral perindopril treatment (5 mg/day) and the control
group received standard DHF treatment alone. None of the
patients was using ACEIs or ARBs before enrollment in the study.
The study was designed as a randomised and prospective study.
All patients underwent a detailed clinical examination
and comprehensive TTE (including TDE) using standardised
equipment (Vivid 3 Pro Echocardiography, General Electric
Corp, Milwaukee, WI, USA). EF was measured both visually and
using the M-mode method. All echocardiographic parameters
were measured and recorded.
Blood pressure measurements were taken after 10 minutes of
rest. The height and weight of all patients were measured and
body mass index (BMI) was calculated. The functional capacity
of the patients was assessed according to the NYHA classification
system (I-IV). Blood samples were drawn from all patients for
biochemistry, complete blood count and NT-proBNP analyses.
Blood samples for NT-proBNP measurements were stored in a
–70°C refrigerator until the time of analysis, and were analysed
using Siemens Corp Immulite-2000 NT-proBNP kites.
The study was approved by the local ethics committee of
IAERH and was conducted in accordance with the Declaration
of Helsinki.
The patients were followed for a mean period of 11 months
(range: three to 16 months). After one month of follow up, blood
samples were drawn from all patients for biochemistry analyses
and complete blood counts. No significant deteriorations were
noted in serum creatinine or potassium levels, and all patients
had tolerated the study drug during this period. Accordingly,
perindopril dose in the study group was up-titrated to 10 mg/day
at the end of one month.
Over the 11-month follow-up period, seven patients decided
to withdraw from the study and three patients died. Perindopril
was discontinued in 10 patients for different reasons (due
to any side effect). Therefore, at the end of the follow-up
period, the perindopril group consisted of 37 patients and the
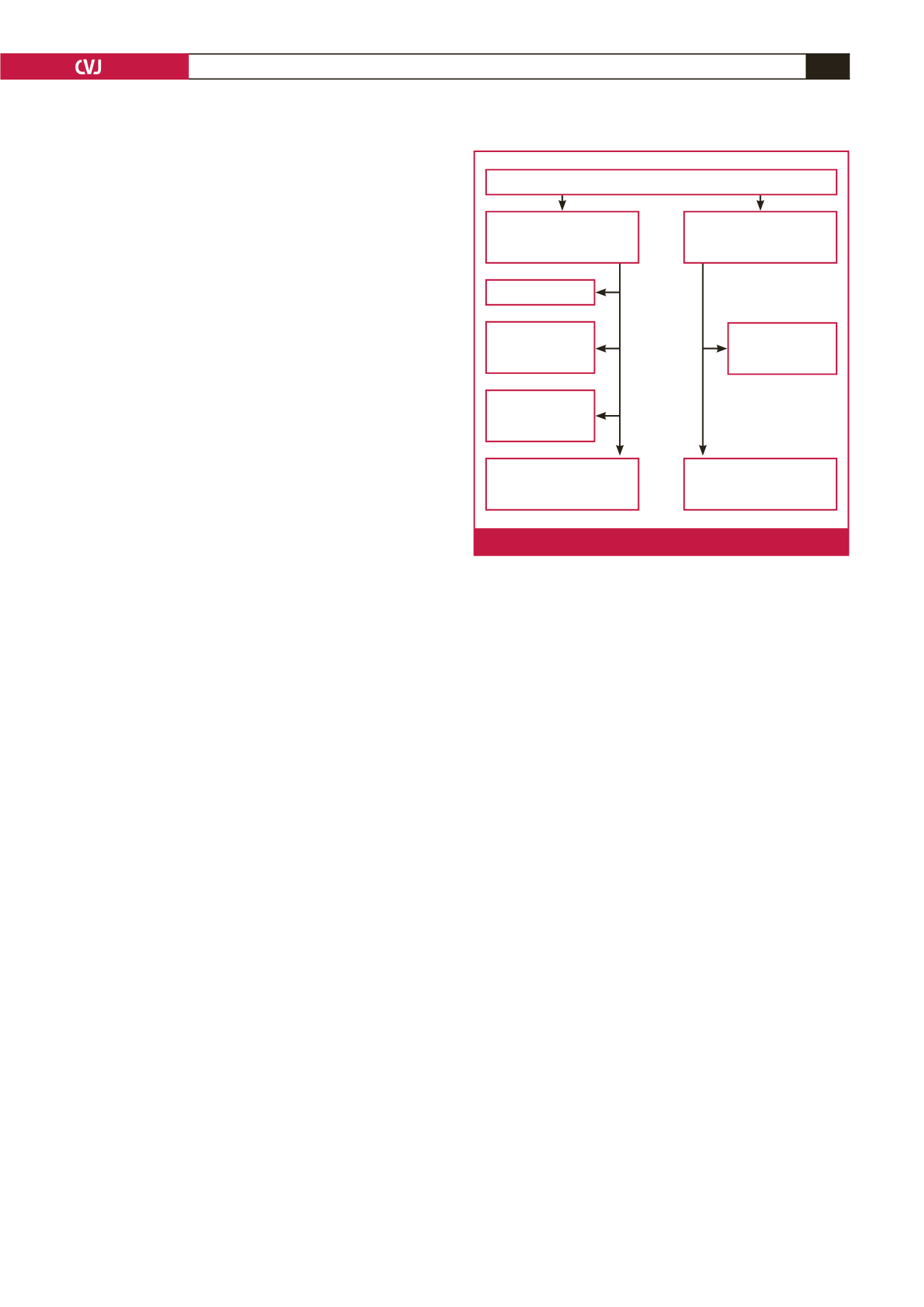
control group included 51 patients (Fig. 1). At the end of the
follow-up period, all patients were invited to the hospital and
echocardiographic assessments were repeated. Blood samples
were drawn for biochemistry and NT-proBNP measurements.
NYHA functional classes were reassessed and recorded.
The primary endpoints of the study were the changes in E
′
,
A
′
, and Sm velocities, E/E
′
, E/A, and E
′
/A
′
ratios, isovolumic
relaxation time (IVRT) and deceleration time (DT) at the end of
the follow-up period. Secondary endpoints included the changes
in NT-proBNP levels and NYHA functional classes.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version
15.0 software program was used for statistical analysis. The
Kolmogorov–Smirnov test was used to determine whether the
numerical variables were normally distributed. A
t
-test was
used to compare normally distributed variables between the
two groups and the Mann–Whitney
U
-test was conducted for
non-normally distributed variables. For categorical variables,
cross-tables were made, and chi-squared analysis or Fisher’s exact
test was performed. The changes from baseline until the end of
the 11-month follow-up period were assessed using the Wilcoxon
test for non-normally distributed variables and a paired-samples
t
-test for normally distributed variables. A
p
-value of
<
0.05 was
accepted as statistically significant.
108 patients were randomised
54 patients were included
in the perindopril group
(Group 1)
At the end of the follow-up
period, perindopril group
consisted of 37 patients
3 patients died
4 patients decided
to withdraw from the
study
3 patients decided
to withdraw from the
study
10 patients
discontinued
perindopril
54 patients were included in
the control group not taking
perindopril (Group 2)
At the end of the follow-
up period, control group
consisted of 51 patients
Fig. 1.
Flow-chart of the study.



















