
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 31, No 6, November/December 2020
340
AFRICA
in the emergency room; thereafter, a bilateral ventricular assist
device (Bi-VAD) was implanted to provide cardiogenic shock
after CPR.
Case report
A 28-year-old man with a history of type 1 diabetes mellitus
and inadequate compliance with insulin administration was
referred to our emergency department due to general weakness
with impaired consciousness lasting one day. Laboratory data
revealed hyperketonaemia (blood ketone level 7.6 mmol/l),
hyperglycaemia [glucose level 1 091 mg/dl (60.55 mmol/l)] and
diabetic ketoacidosis (serum bicarbonate level 6.8 mmol/l).
Additionally, leukocytosis (white blood cell count 20.90
×
10
3
cells/µl) and hyperkalaemia (K
+
5.3 mmol/l) were noted.
Under suspicion of diabetic ketoacidosis, an insulin pump
(insulin actrapid 50 units usage in 500 ml normal saline) was
immediately administered at a rate of 60 ml/h. However,
cardiac arrest occurred abruptly. An electrocardiogram revealed
pulseless VT (Fig. 1) and CPR was immediately performed
with sequential defibrillation, which was repeated five times.
Laboratory data revealed severe hypokalaemia (K
+
1.6 mmol/l).
Large-dose inotropes including dopamine (17.3 mcg/kg/min)
and norepinephrine (26.5 mcg/kg/min) were administered.
Simultaneously, continuous KCl infusion was performed.
However, the haemodynamic status remained inadequate with
refractory VT and low cardiac output.
Peripheral VA-ECMO implantation was therefore performed
through the right femoral vein and artery at a pump speed of
3 000 rpm and flow rate of 3.3 l/min. AGlasgow coma scale result
of E2M2Vt was observed. Blood pressure was approximately
70/60 mmHg irrespective of the high doses of inotropes, and
occasional VT was noted despite anti-arrhythmia medication.
Moreover, echocardiography revealed generalised hypokinesia
of the bilateral ventricles with left ventricular ejection fraction
of 10–15%. However, despite the VA-ECMO support, the patient
developed multiple organ dysfunction, including acute kidney
injury, congestive liver and severe pulmonary oedema.
We therefore changed the VA-ECMO to a temporary
continuous-flow Bi-VAD (Levitronix
®
CentriMag) for better
systemic perfusion (Fig. 2). Using a sternotomy and under
the guidance of transoesophageal echocardiography, the left
ventricular assist device (L-VAD) inflow tube was inserted from
the right superior pulmonary vein into the left ventricular apex,
whereas the outflow tube was cannulated on the ascending aorta.
The right VAD (R-VAD) inflow tube was inserted into the right
atrium, and the outflow tube was inserted into the pulmonary
artery. The operation time was approximately two hours. The
initial L-VAD pump speed was 3 700 rpm and flow rate was 4.74
l/min. The R-VAD pump speed was 3 000 rpm and flow rate was
4.87 l/min (Table 1).
For severe hypoxaemia resulting from pulmonary oedema,
an oxygenator was inserted into the L-VAD outflow to optimise
systemic oxygenation. Mean arterial pressure (MAP) was
maintained at 75–80 mmHg with low-dose norepinephrine (4.3
mcg/kg/min). Potassium level was maintained within the range
4.2–4.7 mmol/l and serum glucose level within 180–220 mg/dl
(9.99–12.21 mmol/l).
At the time of maintaining support with Bi-VAD, the
ventilator was set at 40%
Fi
O
2
with positive end-expiratory
pressure at 8 cmH
2
O to prevent alveolar collapse. The support
pressure was set at 12–15 cmH
2
O to achieve an optimal tidal
volume status (6–8 ml/kg), and the plateau pressure was
controlled under 24 cmH
2
O. During the time of support with
VAD, the patient’s MAP was closely monitored and both VAD
and inotropic agents were gradually tapered down to prevent
vasoconstriction in the vital visceral organs.
Systemic heparinisation was performed to maintain an active
clotting time of 140–160 seconds to prevent thromboembolism.
Additionally, a broad-spectrum antibiotic was prophylactically
prescribed following the Bi-VAD implantation. On day three of
Bi-VAD implantation, the pulmonary oedema was completely
resolved; subsequently, the oxygenator was taken down from
the L-VAD outflow. Although renal function did not recover
immediately, it recovered completely after hospitalisation with
temporary haemodialysis (post-VAD implantation days one
to nine). Following 12-day support with the Bi-VAD, the
Fig. 1.
Electrocardiogram demonstrating refractory ventricular
tachycardia despite correction for profound hypokalaemia.
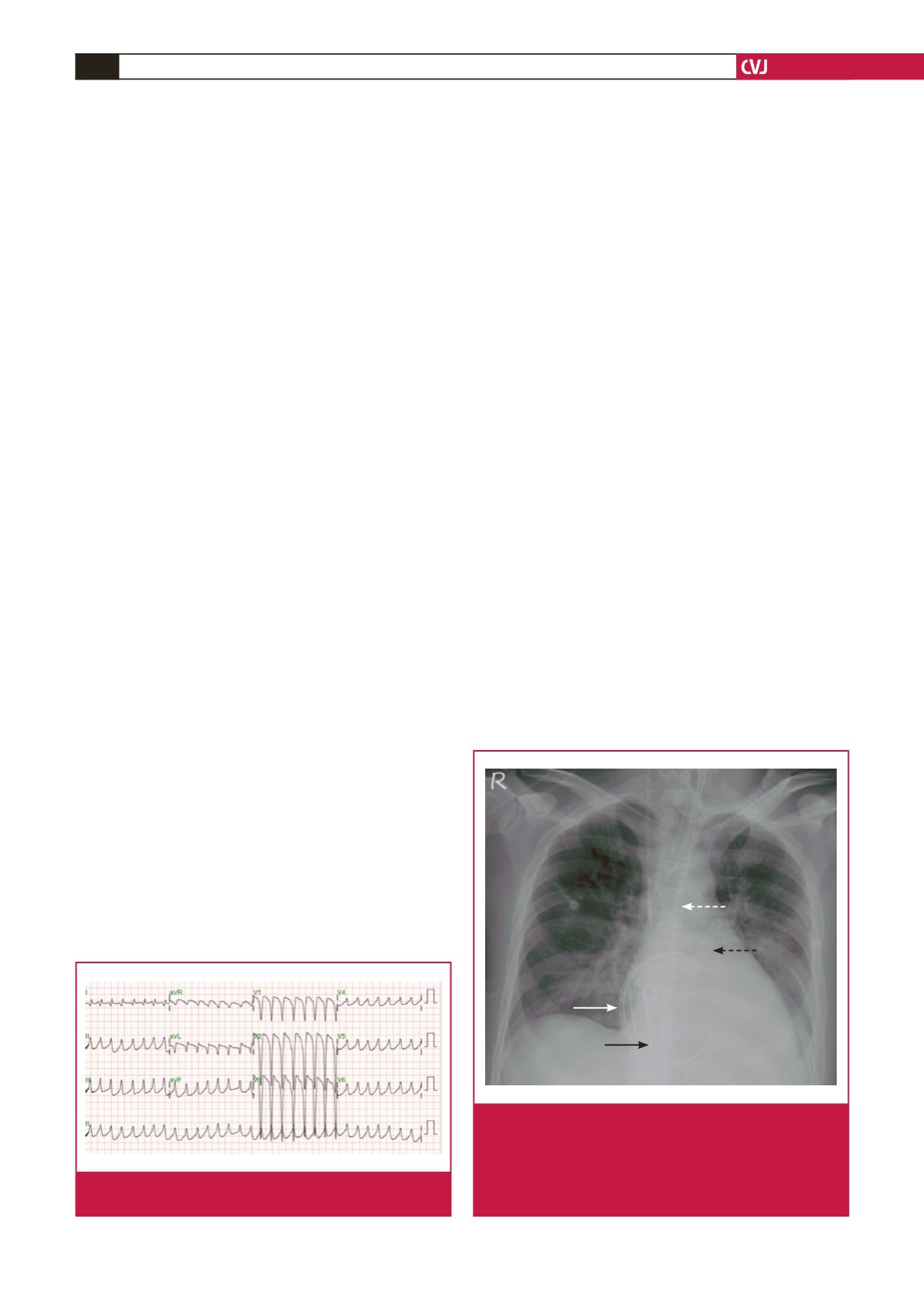
Fig. 2.
The chest plain film demonstrates the L-VAD inflow
tube from the right superior pulmonary vein (solid white
arrow), outflow tube into the ascending aorta (dotted white
arrow), R-VAD inflow tube from the right atrium (solid black
arrow), and outflow tube into the pulmonary artery (dotted
black arrow).



















