CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 4, July/August 2010
AFRICA
235
High-risk patients benefit most from nifedipine GITS–telmisartan
combination
Two very effective antihypertensive medi-
cations with well-established and signifi-
cant cardiovascular outcome studies have
been combined and used for the first
time in early combination therapy in the
TALENT study. Results from this multi-
centre, prospective, randomised, double-
blind trial were announced at the 2010
European Society of Hypertension (ESH)
congress held in June in Oslo, Norway,
and highlighted the rapid, safe and effec-
tive blood pressure-lowering action of this
combination.
1
The TALENT (S
t
udy ev
al
uating
E
fficacyof
N
ifedipineGITS–
T
elmisartan
combination in Blood Pressure Control
and Beyond: Comparison of Two studies)
enrolled 405 patients with office systo-
lic blood pressure at a baseline of
≥
135
mmHg and with a high cardiovascular
risk because of diabetes, the metabolic
syndrome, and echocardiographic/ECG
evidence of left ventricular hypertrophy
or microalbuminuria. Patients could be
admitted to the trial if other antihyper-
tensive medication (ACE inhibitors, other
ARBs, or CCBs) could be safely with-
drawn.
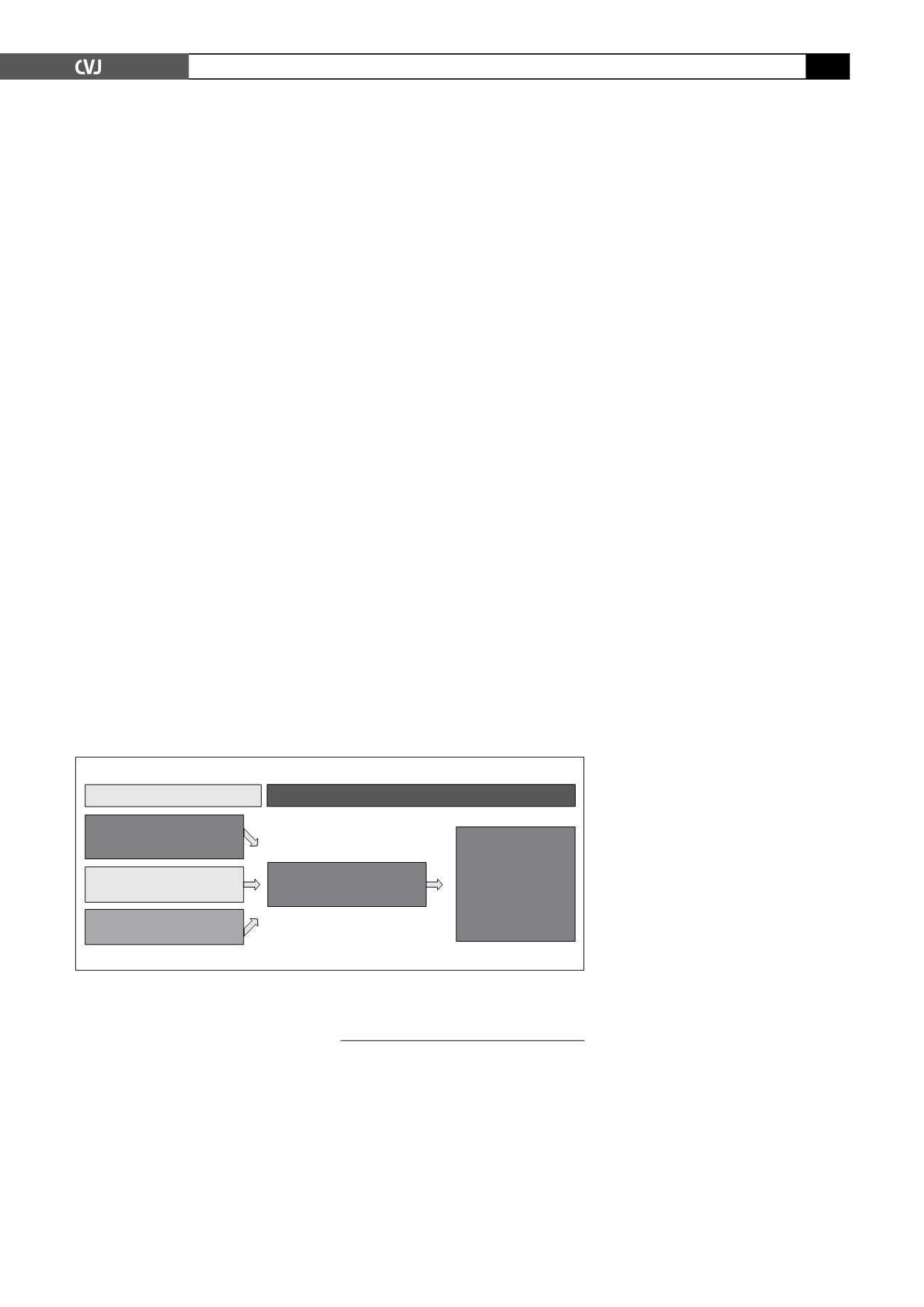
Patients were randomised to initial
administration of telmisartan (80 mg/day)
plus nifedipine GITS (20 mg/d), telmisar-
tan alone, or nifedipine GITS alone in
a 2:1:1 ratio. Treatment was continued
for 24 weeks, shifting the monotherapy
groups to combination therapy after eight
weeks (Fig. 1).
Office and ambulatory blood pressure
was measured after two, eight, 16 and 24
weeks and after eight, 16, and 24 weeks,
respectively. Up-titration occurred when
needed but not to blood pressure levels
below 120 mmHg.
Results
Initiating treatment with the combination
therapy resulted in earlier blood pressure
control. This was maintained throughout
the study period both with regard to office
and ambulatory blood pressure control,
which was reduced by 14.2/3 mmHg and
10/4.7 mmHg, respectively.
Both combination and monotherapy
substantially lowered systolic and diasto-
lic blood pressure. The 24-hour data
showed that the effect was consistent
throughout the 24-hour period. Of impor-
tance is that longer-term control was simi-
lar, irrespective of the initial monotherapy
treatment strategy followed or whether
the combination was initiated first.
In terms of the evidence-based reduc-
tion of cardiovascular outcomes, telmisar-
tan in the ONTARGET
2
studies and nifed-
ipine GITS in the ACTION,
3
INSIGHT,
4
and ENCORE
5
trials have best-in-class
results. This evidence, together with the
South African and international guide-
lines’ emphasis on the use of early effec-
tive antihypertensive agents in high-risk
patients, raises the importance of the
TALENT results in everyday clinical
practice.
J Aalbers, Special Assignments Editor
1. Mancia G, Parati G, Bilo G, Ruilope L on
behalf of the TALENT investigators. Early
blood pressure control by the nifedipine
GITS/telmisartan combination. Abstract.
ESH, Oslo, 2010.
2. The ONTARGET investigators. Telimsartan,
ramipril, or both in patients at high risk of
vascular events.
N Engl J Med
2008;
358
:
1547–1559.
3. Poole-Wilson PA, Lubsen J, Kirwan BA,
et al
. Effect of long-acting nifedipine on
mortality and cardiovascular morbidity in
patients with stable angina requiring treat-
ment (ACTION trial): randomised controlled
trial.
Lancet
2004;
364
: 849–857.
4. Brown MJ, Palmer CR, Castaigne A,
et
al
. Morbidity and mortality in patients
randomised to double-blind treatment with
a long-acting calcium-channel blocker or
diuretic in the International Nifedipine GITS
study: Intervention as a Goal in Hypertension
Treatment (INSIGHT).
Lancet
2000;
356
:
366–372.
5. ENCORE I study group. Effect of nifed-
ipine and cerivastatin on coronary endothe-
lial function in patients with coronary artery
disease: the ENCORE I study (Evaluation of
Nifedipine and Cerivastatin On Recovery of
coronary Endothelial function).
Circulation
2003; 107: 422–428.
Fig. 1. TALENT study design.
0 weeks
8 weeks
16 weeks
24 weeks
Double-blind treatment
Double-blind treatment
Nifedipine GITS 20mg
+ telmisartan 80mg
Nifedipine GITS 20mg
Telmisartan 80mg
Nifedipine GITS 20mg
+ telmisartan 80mg
Optional 8-week
extension of
combination
therapy with
up-titration of
nifedipine GITS
as required
Mancia G. The TALENT Study,
European Cardiology.
2008; 4: 1.
Litha Healthcare Group Ltd has acquired balance of shares in Pharmafrica
With the concluding of the final signa-
tories, Litha Healthcare Group Ltd
has acquired the balance of shares in
Pharmafrica that were not owned by
the group. Four years ago the company
purchased a 26% stake in Pharmafrica as
part of that company’s equity drive.
Pharmafrica markets branded ethical
specialities and over-the-counter pharma-
ceutical products including brands such
as Ecotrin and DS-24.
Litha Healthcare Group Ltd is a JSE-
listed company with diversified opera-
tions in biotechnology, pharmaceutical
and medical devices. The acquisition of
Pharmafrica forms part of the group’s
strategy to grow its pharmaceutical divi-
sion.


