CARDIOVASCULAR JOURNAL OF AFRICA • Vol 21, No 4, July/August 2010
AFRICA
227
and LVIDs) were 45 mm (37–56 mm) and 30 mm (23–35 mm),
and left ventricular ejection fraction (LVEF) was 64% (
>
50%).
After admission, the patient was treated with potassium and
magnesium supplementation and beta-blocker therapy. During
an intra-cardiac electrophysiological examination, repeated right
atrial and ventricular stimulation did not evoke any atrial arrhyth-
mia, ventricular tachycardia or ventricular fibrillation.
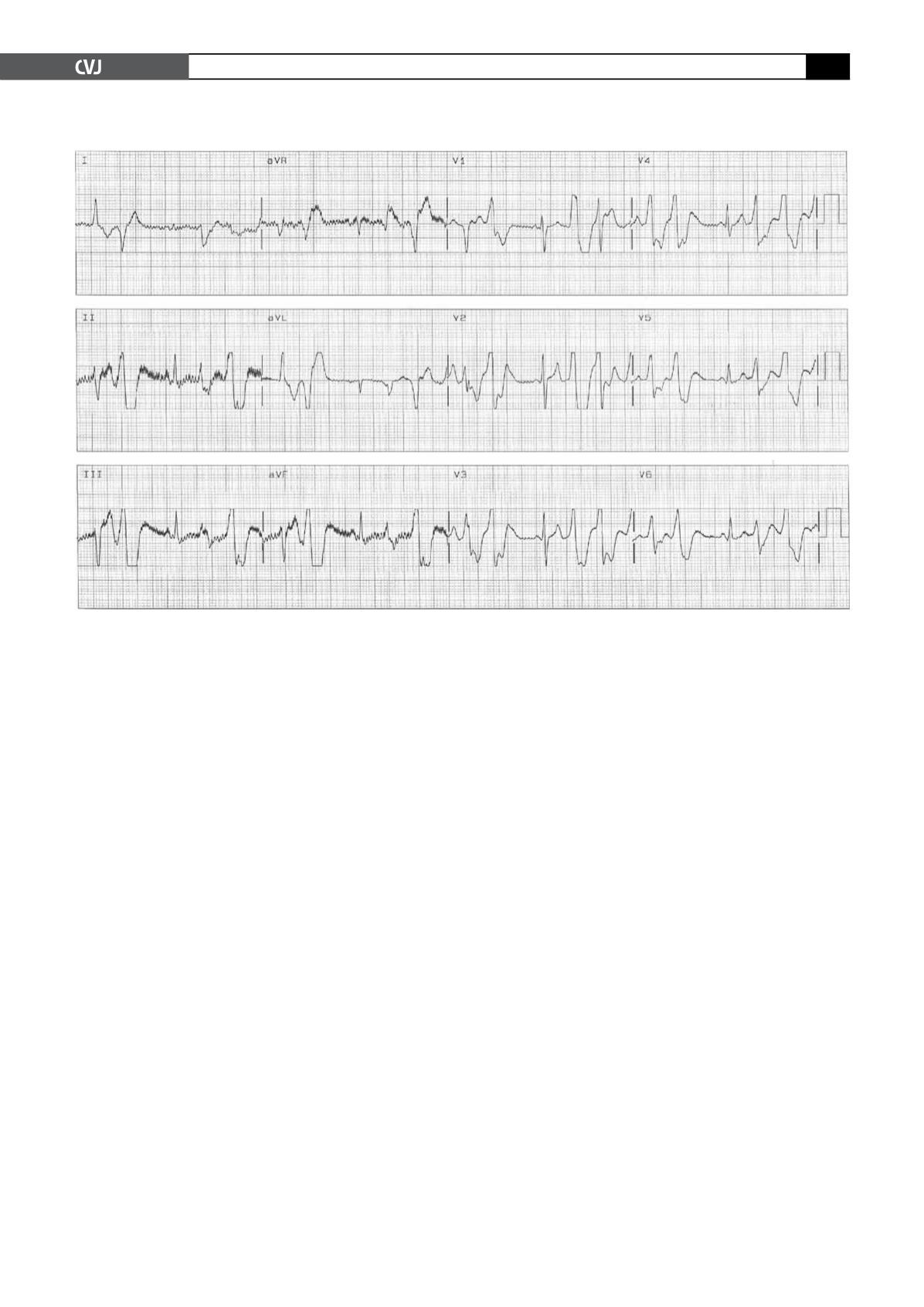
Whenever the ventricular rate increased to more than 100
beats/min, frequent premature ventricular contractions and short
episodes of polymorphic ventricular tachycardia were observed
(Fig. 3). All of these episodes terminated spontaneously after a
few minutes. The diagnosis of CPVT was made.
It was decided to perform ICD implantation without radio-
frequency ablation. Postoperatively, the patient continued taking
a beta-blocker orally. Six months after ICD implantation, pace-
maker programming revealed two appropriate ICD discharges
(Fig. 4). As the patient had previously been diagnosed with anxi-
ety disorder and panic attacks, Seroxat (SSRI) 20 mg daily was
added, and during a two-year follow-up period, no further ICD
discharges occurred.
Discussion
CPVT is an arrhythmic disorder with a high fatality rate. The
incidence of adverse events, including syncope, ventricular
tachycardia and ventricular fibrillation at 40 years of age is about
80%,
2
and between 20 and 30 years, the incidence of sudden
cardiac death is 30 to 50%.
3
Currently, there are two known
genetic mutations in CPVT, namely, RyR2 and CASQ2.
4
These
two ion channels mediate the transportation of calcium ions
from the sarcoplasmic reticulum into the cytoplasm. Mutations
in these two genes lead to intracellular calcium overload as the
basis for the subsequent arrhythmias.
Sympathetic stimulation may lead to delayed after-depolari-
sations (DAD) and triggered activity, which will also induce
arrhythmias. Cerrone
et al
.
5
demonstrated that anesthetised mice
with RyR2 mutations had a higher incidence of CPVT than in the
isolated heart, suggesting that sympathetic stimulation may have
an impact on CPVT.
Beta-blockers are the first-line agents for the treatment of
CPVT. They are used to prevent episodes of ventricular tachycar-
dia. However, the efficacy of beta-blockers in CPVT is inferior
when compared to LQT1 syndrome (KCNQ1 gene mutation),
but is comparable to the LQT2 syndrome. Even if anti-adrenergic
drugs areused, there is still a suddendeath rateof 10%inCPVT, and
50% of patients taking beta-blockers may need ICD implantation.
For patients who experience episodes of ventricular tachycar-
dia during an exercise stress test despite taking beta-blockers,
as well as patients who still experience syncope attacks after
taking the maximum load of beta-blockers, ICD implantation is
recommended. There are also reports on the treatment of CPVT
by cardiac sympathectomy.
6
For patients experiencing recurrent
ICD discharges after ICD implantation, or patients with poor
compliance in taking beta-blockers, cardiac sympathectomy may
be considered.
The physiological basis for the beneficial effect of cardiac
sympathectomomy is as follows: as cardiac sympathectomy can
reduce local norepinephrine release by the cardiac sympathetic
nerves in the myocardium, malignant ventricular arrhythmias
caused by the adrenergic stimulant action may be reduced.
ICD implantation does not reduce the occurrence of cate-
cholaminergic polymorphic ventricular tachycardia events.
Appropriate and inappropriate ICD discharges are often caused
by exercise, leading to a decline in the quality of life. Moreover,
Fig. 3. Premature ventricular contractions and short episodes of PVT occurred after intravenous infusion of isoprot-
erenol.


