
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 6, November/December 2015
230
AFRICA
To pretest the resulting application, we used the sample data
available in the WHF sample database.
16
We entered the values
from the sample database into our newly developed electronic
data-collection forms, running on two different mobile devices:
a general purpose seven-inch tablet running on the Android
operating system (purchased commercially for US$59.99) and a
general-purpose Android smartphone (purchased commercially
for US$29.99). We extracted the data from CommCare into our
copy of the WHF database, reviewed the data and the reports
against the original WHF sample database, and confirmed their
being identical.
Field testing
A version of the eRegister was adapted to the specific needs of a
school-based RHD screening programme in Lusaka, Zambia, in
which health workers conducted clinical and echocardiographic
assessment of schoolchildren in order to detect those with
previously unrecognised RHD. Eight health professionals
(including local nurses and radiographers, and programme
management staff) were orientated on the use of the eRegister
over two half-day training sessions and then received ongoing
support as needed to utilise the tool.
The eRegister was deployed to support screening of 261
children in pilot screening sessions conducted from June to
November 2014, and 1 022 children in full-scale screening that was
conducted during February and March 2015. The mobile devices
used were Samsung Galaxy Tab 2 tablets, which were sourced
locally. Data were entered into the eRegister using a combination
of tablets and laptops on site at schools and at the referral hospital.
Results
The resulting eRegister application enables simultaneous data
collection and entry. For example, field workers can directly enter
patient data into the system’s electronic data-collection forms,
which then automatically populate the cloud-based database,
using either handheld mobile devices or computer terminals in
clinics and hospitals. When data are entered on mobile devices,
synchronisation with the central database takes place securely
the next time a cellular or internet connection is established (i.e.
wireless connection is not necessary at the time of data entry).
Thus the database is continually updated, which streamlines its
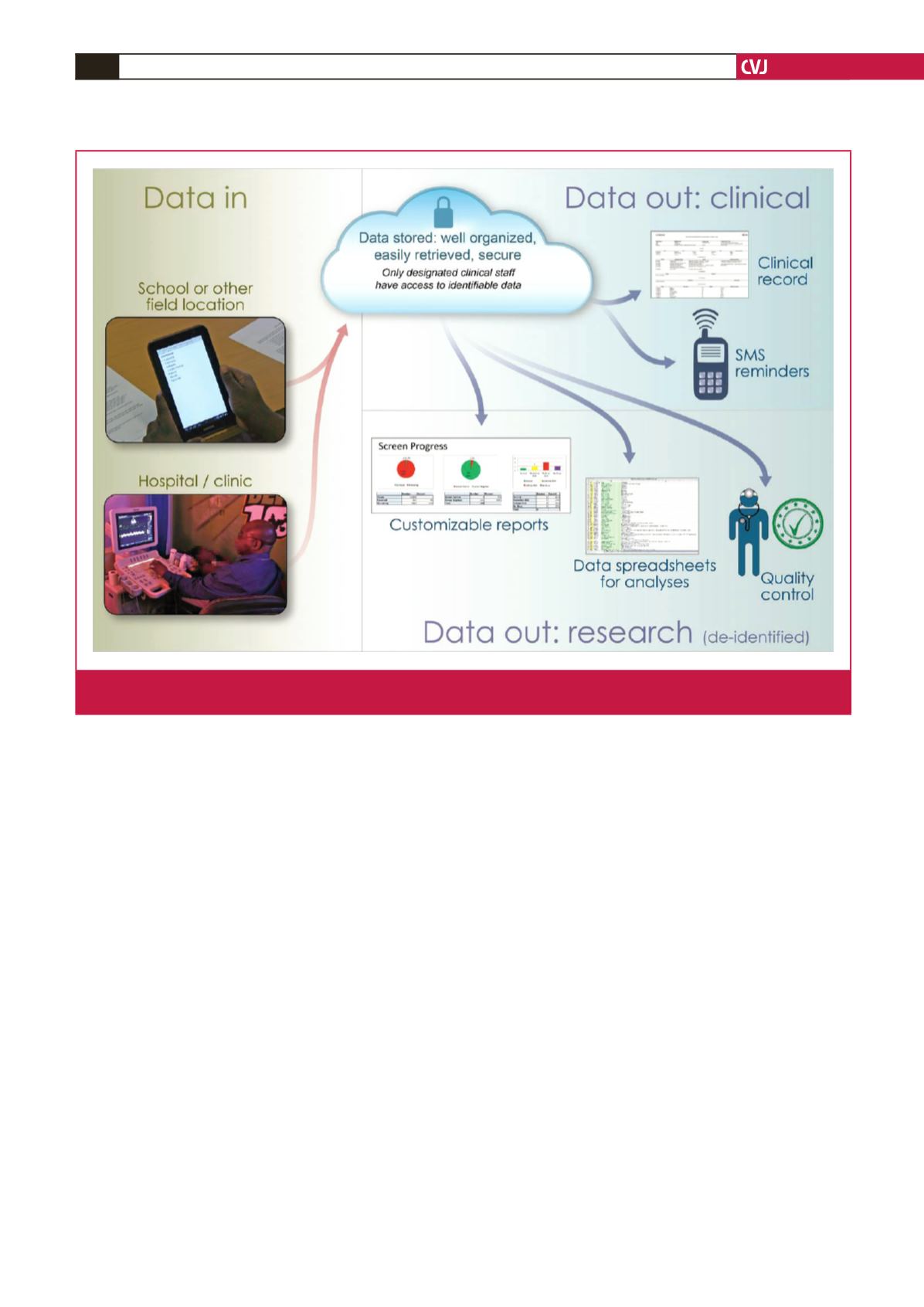
various clinical and research functions (Fig. 3).
The eRegister variables match those in the WHF register.
Variables relating to existing data fields (e.g. names of villages or
clinics) can easily be tailored to local needs and new data fields
can be added. The system application itself can be installed to
mobile devices by downloading from
https://sites.google.com/site/rhderegister/home.
All access to the CommCare platform including mobile
submissions is achieved through Hypertext Transfer Protocol
Secure (HTTPS) and is cryptographically secure. Data stored in
the eRegister is confidential and password-protected at all times.
Fig. 3.
Overview of the targeted eRegister system. Data are collected through electronic forms on a variety of devices, securely
managed in the cloud, and made accessible to multiple users for varying clinical care and clinical research purposes.

















