
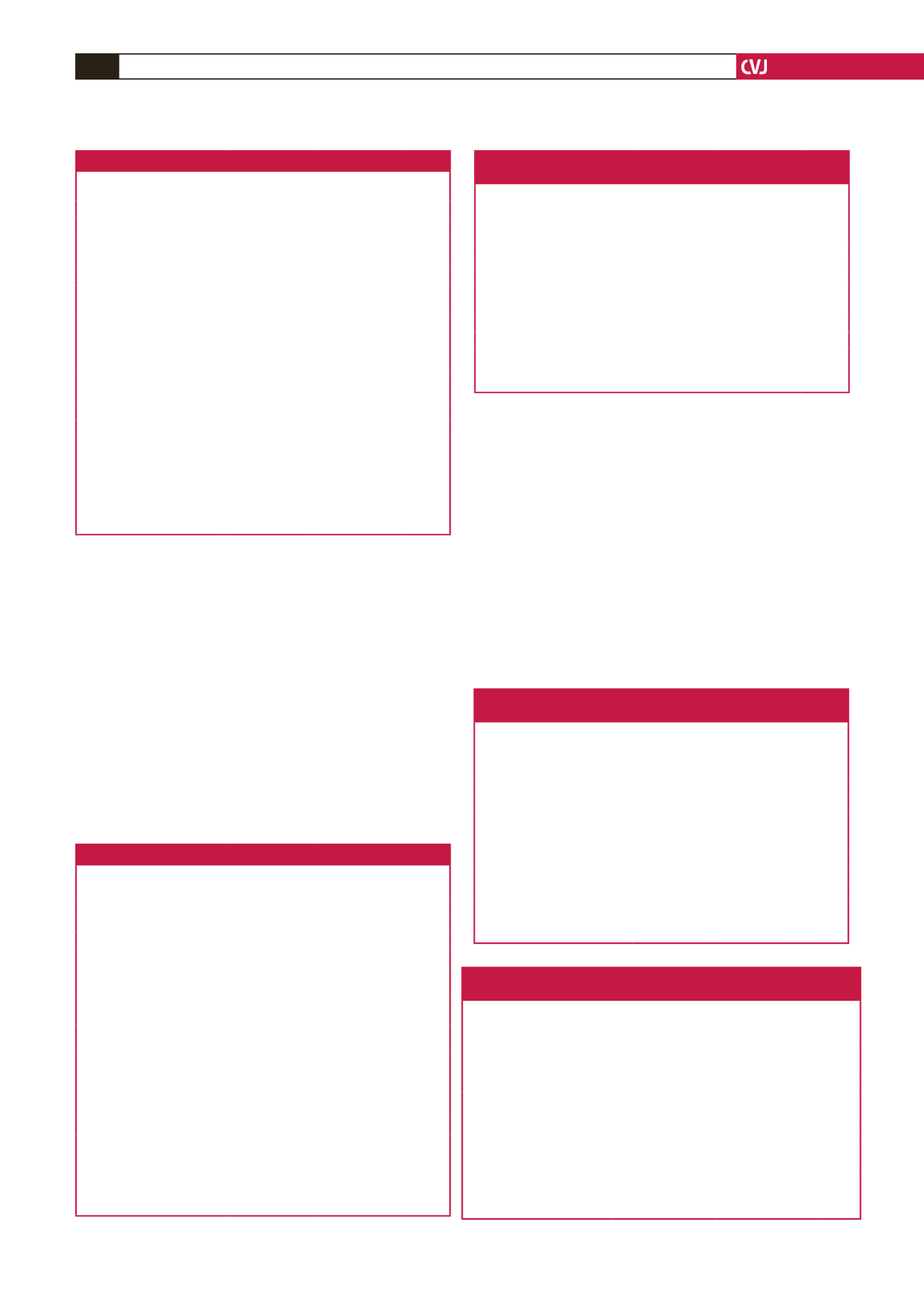
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 3, May/June 2018
164
AFRICA
statistically significant difference. However, a significant
difference was observed in hyperaemia diameter in favour of
rosuvastatin (Table 4).
Percentage changes in non-invasive brachial artery
measurements after 12 months of treatment were compared
between the two groups. A statistically significant difference was
found in percentage change in the rosuvastatin group’s brachial
artery post-nitrate diameter (
p
<
0.05). Non-significant changes
were found in the basal diameter and hyperaemia velocity in
favour of the rosuvastatin group (
p
=
0.089 and
p
=
0.088,
respectively) (Table 5).
Discussion
This study revealed that both atorvastatin and rosuvastatin had
an effect on baseline lipid values, brachial artery basal diameter
and hyperaemia diameter, and FMD and EID measurements.
Comparing 12-month non-invasivemeasurements of atorvastatin
and rosuvastatin groups, it was found that the statins had
similar effects on endothelial function in the subjects with
hyperlipidaemia.
Post-nitrate diameter in the rosuvastatin group was
significantly improved at the end of the 12-month treatment
compared to baseline values. Endothelial dysfunction is one of
the early functional markers of atherosclerosis.
11,12
Preventative
measurements should be taken before clinical manifestation
of atherosclerotic events. For this reason, detection of early
atherosclerotic changes is of great importance in reducing risk
factors. Endothelial dysfunction can be detected via FMD, a
Table 1. Post-treatment versus baseline values in the atorvastatin group
Atorvastatin
Baseline
mean
±
(SD)
12-month
mean
±
(SD)
p
-value
*
Basal diameter (mm)
4.0
±
0.6
4.1
±
0.6
0.045
Hyperaemia diameter (mm)
4.2
±
0.6
4.3
±
0.6
0.436
NTG diameter (mm)
4.5
±
0.6
4.7
±
0.6
0.002
FMD (%)
8.5
±
3.3
10.4
±
4.1
<
0.001
EID (%)
15.5
±
5.1
16.3
±
4.8
0.143
TC (mg/dl)
(mmol/l)
261.3
±
28.3
(6.77
±
0.73)
174.3
±
38.9
(4.51
±
1.01)
<
0.001
TG (mg/dl)
(mmol/l)
161.8
±
66
(1.83
±
0.75)
131.9
±
50
(1.49
±
0.57)
<
0.001
LDL-C (mg/dl)
(mmol/l)
176.8
±
23.5
(4.58
±
0.61)
92.9
±
28.1
(2.41
±
0.73)
<
0.001
HDL-C (mg/dl)
(mmol/l)
54.7
±
12.1
(1.42
±
0.31)
54.4
±
12.4
(1.41
±
0.32)
0.145
AST (U/l)
23.8
±
9.1
21.9
±
5.6
0.068
ALT (U/l)
23.6
±
12.8
24.1
±
13.1
0.746
CPK (U/l)
136.8
±
74
113.4
±
67
0.427
*Student’s
t
-test,
p
<
0.05.
SD: standard deviation; NTG: post-nitrate; FMD: flow-mediated dilation; EID:
endothelium-independent dilation; AST: aspartate transaminase; ALT: alanine
transaminase; CPK: creatinine phosphokinase; TG: triglycerides; TC: total
cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density
lipoprotein cholesterol.
Table 2. Post-treatment versus baseline values in the rosuvastatin group
Rosuvastatin
Baseline
12 months
p
-value
*
Basal diameter (mm)
4.0
±
0.5
4.2
±
0.5
0.003
Hyperaemia diameter (mm)
4.4
±
0.5
4.6
±
0.5
<
0.001
NTG diameter (mm)
4.6
±
0.5
4.7
±
0.5
0.687
FMD (%)
9.7
±
3.4
12.7
±
3.7
<
0.001
EID (%)
16.8
±
5.8
18.2
±
5.8
0.105
TC (mg/dl)
(mmol/l)
271.2
±
35.7
(7.02
±
0.92)
188.4
±
44.8
(4.88
±
1.16)
<
0.001
TG (mg/dl)
(mmol/l)
173.5
±
55.2
(1.96
±
0.62)
143
±
54.1
(1.62
±
0.61)
<
0.001
LDL-C (mg/dl)
(mmol/l)
180.5
±
26.1
(4.67
±
0.68)
105
±
39.2
(2.72
±
1.02)
<
0.001
HDL-C (mg/dl)
(mmol/l)
56.8
±
13.6
(1.47
±
0.35)
54
±
11.5
(1.40
±
0.30)
0.093
AST (U/l)
22.8
±
6.7
23.3
±
6.5
0.819
ALT (U/l)
22.8
±
9.7
23.6
±
9.3
0.759
CPK (U/l)
94
±
31.3
116
±
73.8
0.007
*Student’s
t
-test,
p
<
0.05.
SD: standard deviation; NTG: post-nitrate; FMD: flow-mediated dilation; EID:
endothelium-independent dilation; AST: aspartate transaminase; ALT: alanine
transaminase; CPK: creatinine phosphokinase; TG: triglycerides; TC: total
cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density
lipoprotein cholesterol.
Table 3. Statistical comparison between atorvastatin and rosuvastatin
groups in terms of baseline brachial artery measurements
Baseline brachial artery
measurements
Atorvastatin
group
Rosuvastatin
group
p
-value
*
BA basal diameter (mm)
4.01
±
0.6
4.02
±
0.5
0.850
BA basal velocity (cm/s )
71.95
±
14.8
79.32
±
16.9 0.240
BA hyperaemia diameter (mm)
4.34
±
0.6
4.43
±
0.5
0.404
BA hyperaemia velocity (cm/s)
72.21
±
15.9
73.4
±
16.3 0.713
BA NTG diameter (mm)
4.6
±
0.6
4.69
±
0.5
0.451
BA NTG velocity (cm/s)
68.92
±
15.4
68.23
±
15.5 0.833
BA FMD (%)
8.52
±
3.3
9.71
±
3.4
0.750
BA EID (%)
15.31
±
5.1
16.84
±
5.8
0.159
*Student’s
t
-test,
p
<
0.05.
SD: standard deviation; BA: brachial artery; NTG: post-nitrate; FMD: flow-
mediated dilation; EID: endothelium-independent dilation.
Table 4. Rosuvastatin versus atorvastatin in terms of non-invasive test
results after 12 months of statin therapy
Brachial artery measurements
after therapy
Atorvastatin
group
(
n
=
50)
Rosuvastatin
group
(
n
=
54)
p
-value*
BA basal diameter (mm)
3.87
±
0.59
4.17
±
0.54
0.09
BA basal velocity (cm/s)
78.82
±
13.40 72.55
±
22.3
0.743
BA hyperaemia diameter (mm)
4.29
±
0.60
4.60
±
0.52
0.006
BA hyperaemia velocity (cm/s)
80.03
±
16.8
71.12
±
18.5
0.017
BA NTG diameter (mm)
4.78
±
0.60
4.75
±
0.52
0.757
BA NTG velocity (cm/s)
71.2
±
19.45
61.1
±
16.32 0.323
BA FMD (%)
10.42
±
3.3
11.75
±
3.7
0.122
BA EID (%)
16.3
±
4.83
18.2
±
5.83
0.115
*Student’s
t
-test,
p
<
0.05.
SD: standard deviation; BA: brachial artery; NTG: post-nitrate; FMD: flow-
mediated dilation, EID: endothelium-independent dilation.
Table 5. Changes in brachial artery measurements after 12 months of
treatment in the atorvastatin versus rosuvastatin group
Change in brachial artery
measurements after therapy
Atorvastatin
group (
n
=
50)
Median (25
–
75%)
Rosuvastatin
group (
n
=
54)
Median (25–75%)
p
-value
*
BA basal diameter (mm)
0.011 (–0.041–0.031) 0.010 (–0.007–0.045) 0.089
BA basal velocity (cm/s)
0.001 (–0.119–0.157) –0.043 (–0.206–0.378) 0.120
BA hyperaemia diameter (mm) 0.018 (–0.034–0.060) 0.021 (0.011–0.048) 0.644
BA hyperaemia velocity (cm/s) 0.056 (–0.087–0.0347) –0.012 (–0.168–0.116) 0.088
BA NTG diameter (mm)
0.028 (0.008–0.045) 0.020 (–0.028–0.036) 0.045
BA NTG velocity (cm/s)
–0.004 (–0.167–0.113) –0.020 (0.0113–0.088) 0.982
BA FMD (%)
0.203 (0.008–0.441) 0.193 (0.049–0.0433) 0.958
BA EID (%)
0.110 (–0.115–0.225) 0.037 (–0.460–0.347) 0.827
*Mann–Whitney
U
-test,
p
<
0.05.
BA: brachial artery; NTG: post-nitrate; FMD: flow-mediated dilation; EID: endo-
thelium-independent dilation.

















