
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 5, September/October 2019
300
AFRICA
specialist level as severe disorders such as phytosterolaemia may be
overlooked. A register for FH may enhance support for treatment
and allow efficient introduction of new treatment strategies.
Genotype to phenotype in FH (Prof SE
Humphries)
According to many recent guidelines on the identification and
management of FH,
9
all patients with a clinical diagnosis of
FH ought to be investigated for the presence of a pathogenic
mutation in the genes known to cause FH. The purpose of the
genetic testing is not only for confirmation of the diagnosis but
also to support cascade testing of the family. Ideally there should
be a genetic diagnosis together with a phenotypic description
by LDL-C concentration. There is strong evidence that LDL-C
concentration on its own does not accurately discriminate between
affected and unaffected family members of patients with FH;
10
and
accuracy worsens with age, with an unacceptably high rate of false-
positive and false-negative diagnoses based on measuring LDL-C
concentration. Since half of the offspring and siblings of an
index case are expected to inherit the condition, genetic diagnosis
is preferred because it can unambiguously identify carriers of
the monogenic causes of FH who can then be offered early and
effective lipid-lowering treatment along with lifestyle advice.
In patients referred to the lipid clinic with a clinical diagnosis
of FH, an FH-causing mutation can only be found in about
50% of the cases in the UK. Talmud
et al
.
11
therefore examined
the hypothesis that, despite small effects individually on LDL-C
concentration, inheritance of a larger-than-average number
of common LDL-C-raising variants in the genes for the LDL
receptor, apolipoprotein B, PCSK9, apolipoprotein E and
several others, could in combination produce a similar degree
of hypercholesterolaemia to FH.
11
At first, 12 variants were used
in the identification as indicated in Fig. 2 but this was simplified
to include only the six variants having the largest effect.
12
The
polygenic cause appears to explain at least 80% of the FH
patients in whom no monogenic cause could be found. The same
common variants also significantly modify the phenotype of
monogenic FH patients.
Patients with LDL hypercholesterolaemia ascribed to a
polygenic cause were slightly older and had higher plasma
triglyceride levels and the LDL-C concentration was a little
lower.
12
Furthermore, such patients had a lower degree of
atherosclerosis in their carotid arteries as determined by
ultrasound that measured an increased carotid intima–media
thickness. This is presumed to be due to later onset of the
influence of the polymorphic genes in contrast to the higher level
of LDL-C since birth in patients with monogenic FH.
The recognition of polygenic FH is important as it can cut
short extensive and expensive genetic screening in the context
of diagnosing patients, and allows the scarce resources of nurses
and lipid clinic doctors to focus on monogenic families and to
offer cascade testing and maximum lipid-lowering treatment. In
subjects with a polygenic cause, cascade testing will be less cost
effective and the patients themselves can be more easily treated
in general practice. The approach to genetic testing is outlined
in Fig. 3.
Treatment of FH (Prof FJ Raal)
Worldwide, it is estimated that one baby is born with FH
every minute and should be treated to reduce the high risk
of cardiovascular disease. This risk is estimated to be more
than 80-fold for persons in the fourth decade of life when
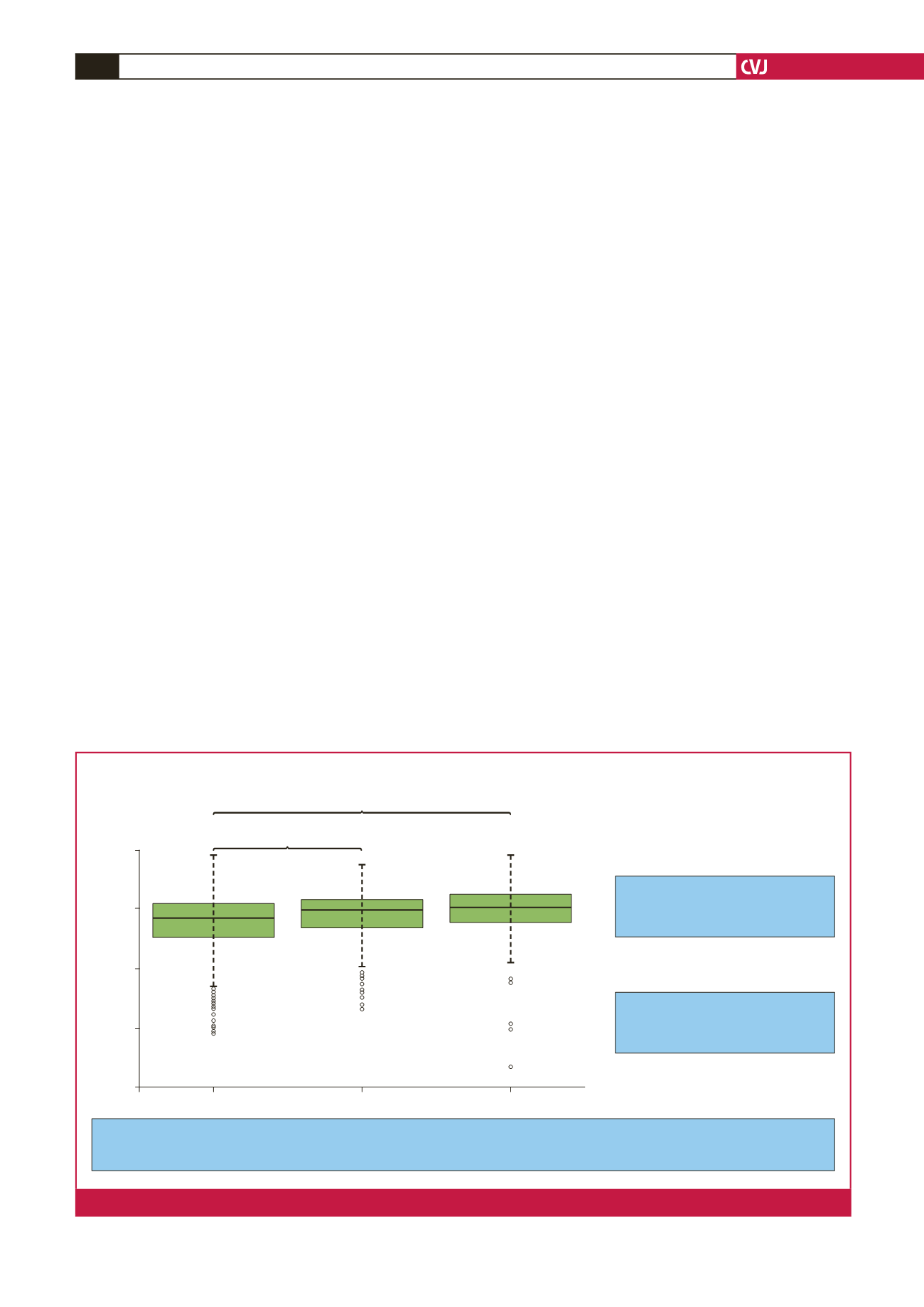
Higher LDL-C gene score in FH/M– group vs controls suggests that, in a significant
proportion of this group, their elevated LDL-C can be explained by a polygenic component
Even where the patient carries an
FH-causing mutation there is a
greater-than-average polygenic background
54% of FH/M– are in the top three deciles
of score vs 11% in the lowest three deciles
Box–Whisker plot of mean weighted 12-SNP score
Talmud
et al. Lancet
2013.
Controls (WHII)
FH with a mutation
FH with no mutation
LDL-C gene score
15
10
5
0
–5
p
=
4.5
×
10
–16
p
=
1.6
×
10
–5
Fig. 2.
A comparison of the gene score for polygenic FH between control, monogene-negative and polygene FH.



















