
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 5, September/October 2019
AFRICA
301
matched for other risk factors with their peers.
13
The impact
of plasma LDL-C on arterial disease is related to the amount
of exposure, a function of concentration and time.
14
Statins
significantly improve the outcome in heterozygous FH,
6
and even
in homozygous FH, where their impact on LDL-C reduction is
less.
15
The addition of ezetimibe to statins results in a significant
further reduction in LDL-C concentration.
Other strategies to lower LDL-C have been researched. Some
drugs such as squalene synthetase inhibitors and thyromimetics
that influence the production of cholesterol and LDL receptor
expression, respectively, have been discontinued owing to
adverse effects. Limiting the production of apolipoprotein B by
antisense oligonucleotides had a significant LDL-C-lowering
effect in homozygous FH
16
and therefore could be of benefit
in heterozygous FH not controlled by more conventional
therapy. Lomitapide, an inhibitor of microsomal triacylglycerol
transfer protein, also limits lipoprotein production but has not
yet been marketed in South Africa. It affects both the liver
and the intestine and can cause significant hepatic steatosis.
The management algorithm is indicated in Fig. 4 and include
the use of older drugs such as niacin and fibrates with lesser
impact.
The powerful effect of PCSK9 on LDL receptor activity
is underscored by the significantly lower plasma LDL-C
concentrations in loss-of-function variants, and the appearance
of the FH phenotype in gain-of-function variants. Paradoxically,
lowering hepatocyte cholesterol content results in an upregulation
of not only HMGCoA reductase and the LDL receptor, but also
increases the expression of PCSK9. Antibodies that prevent
the binding of PCSK9 to the LDL receptor and subsequent
lysosomal degradation of this receptor enhance the recycling of
receptors and consequently enhance clearance of LD-C from
the plasma.
Alirocumab and evolocumab have been approved for
the market internationally but not in South Africa. These
humanised monoclonal antibodies are injected subcutaneously
with tremendous additional effect on LDL-C reduction in
heterozygous FH.
17
As is the case for statins, the efficacy is
generally related to the function of the normal LDL receptor
allele. It is therefore expected that every heterozygous FH
patient, if tolerant of the combination of statin, ezetimibe
and anti-PCSK9 medication, can achieve the target LDL-C
concentration of 3 mmol/l or less.
Despite the effectiveness of statins, FH remains a seriously
under-recognised and therefore under-treated condition. The
estimated proportion of FH subjects identified has been
disappointing, except for the Netherlands (71%).
18
In South
Africa this figure is placed at 1%.
The way forward (Prof PJ Talmud)
The importance of diagnosing FH was emphasised as there is
currently effective control with statins, and additional agents
have been developed when additional treatment is advisable.
Although FH has a long history as a clinical entity and obtained
a mechanistic explanation in the 1970s, genetic explanations
were proved in the LDL receptor gene and apolipoprotein B100.
The story of PCSK9 is remarkable. Within a decade of the
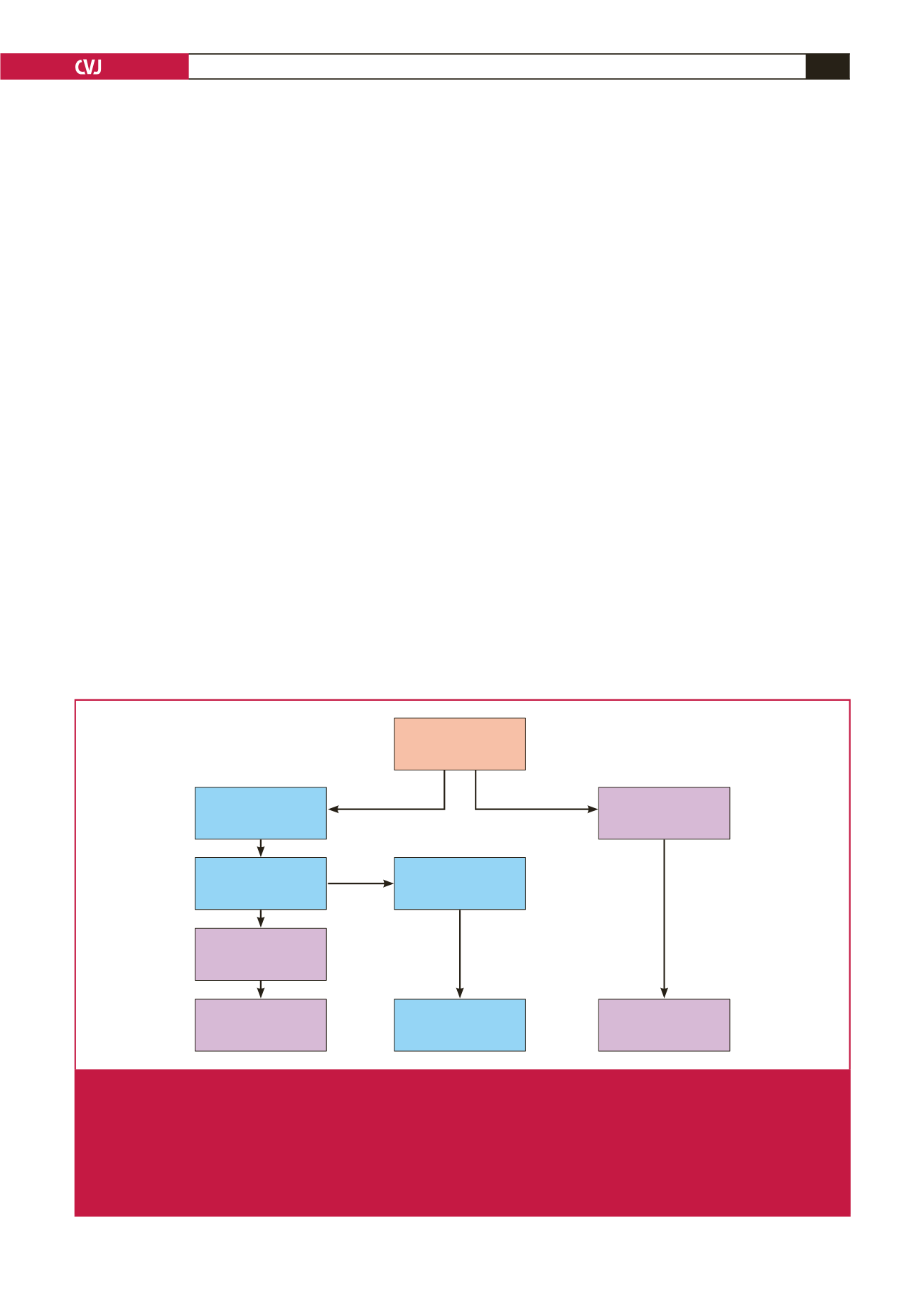
FH patient
LDL-C > 4.9 mmol/l
Fam hist, TX etc
Determine 6-SNP
LDL-C gene score
High probability of
polygenic cause of
high LDL-C
Do not cascade – low
detection of affected
relatives
Low probability of
polygenic cause of
high LDL-C
Continue search for
monogenic cause of
FH
No FH-causing
mutation detected
FH-causing mutation
detected in
LDLR/APOB/PCSK9
Use mutation to
cascade test all
1st-degree relatives
Next gen sequence of FH genes
OR whole-exome sequence
Fig. 3.
The diagnostic approach to FH.
21
After the six-SNP LDL-C gene score has been determined there are two different care
pathways. If the score is in the lowest quintile (i.e. < 20th percentile), a polygenic cause for the patient’s high LDL-C level is
statistically very unlikely. Since mutation in
LDLR
/
APOB
/
PCSK9
has already been ruled out, this suggests that the pheno-
type may be caused by a (monogenic) mutation in a yet-to-be-identified gene, and the patient can usefully be consented
and recruited into a research project to find this gene using whole-exome or whole-genome sequencing. By contrast, if the
score is in the top four quintiles (i.e.
>
20th percentile), the high LDL-C level can safely be assumed to be due to a ‘poly-
genic’ cause (i.e. the inheritance of a greater-than-average number of common LDL-C-raising variants of small effect that in
combination cause the phenotype).



















