
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 31, No 2, March/April 2020
AFRICA
e3
system.
4,6
Cardiac involvement is relatively frequent and one
of the most serious manifestations of EGPA, accounting for
approximately half of the deaths attributable to EGPA. It is
more common in patients with an absence of ANCA and those
with higher eosinophil counts.
2,7-9
Clinical manifestations are
various, including myocarditis, pericarditis, pericardial effusion,
heart failure, arrhythmias, valvular insufficiencies, intra-cardiac
thrombus formation, and others.
7,9
The histological features of EGPA are tissue eosinophilia,
necrotising vasculitis and extravascular eosinophilic granulomas.
However, histological findings may vary according to the organs
involved. Cardiac pathology usually shows endomyocardial and
pericardial eosinophilic infiltration and only rarely, coronary
vasculitis.
1,3,9
Because cardiac involvement in EGPA is relatively
frequent and could be fatal, early detection is important.
2,3,7
Transthoracic echocardiography can show a wide spectrum
of cardiac abnormalities, including systolic dysfunction, valvular
insufficiencies, pericardial effusion and intra-cardiac thrombus.
7,9
Cardiac MRI is the most sensitive technique to evaluate
cardiac involvement in EGPA. It can detect clinically silent
and undisclosed myocardial involvement.
4,7,9
Late gadolinium
enhancement in cardiac MRI suggests active endomyocarditis
or endomyocardial fibrosis. Most enhancing lesions were apical
and mid-cavity segments of the left ventricle.
7,10
Therefore
late gadolinium enhancement of endocardial layers could be
associated with eosinophilic Loeffler-like endocarditis.
7,11
The general consensus for treatment is based on the usage
of systemic glucocorticoids, adding other immunosuppressants
if the prognosis is poor.
3,4
The most commonly used prognostic
tool is the Five Factor Score (FFS) scale. According to this
scale, one point is given for each of the following: cardiac
involvement, severe gastrointestinal manifestation, central
nervous system involvement and renal impairment. Patients with
a good prognosis have a FFS of 0 points and are treated solely
with corticosteroids, while for patients with a poor prognosis
(FFS ≥ 1), consider the addition of immunosuppressants, usually
cyclophosphamide.
4,12
In this presented case, diagnosis was based on the clinical
history and laboratory and pathology results. Hyper-eosinophilic
syndrome (HES) is probably the most challenging differential
diagnosis of EGPA. We could rule out reactive HES from
parasitic infection, allergy and drug reaction by clinical history.
The absence of PDGRFA and PDGFRB gene fusion suggested
it was less likely to be myeloid or lymphoid HES, and idiopathic
HES is rarely accompanied by asthma.
2,3
After ruling out HES,
the diagnosis was made by American College of Rheumatology
(ACR) criteria.
13
Cardiac MRI is known as a sensitive modality to evaluate
cardiac involvement,
4,7,9
however, we could not use it owing to
the patient’s refusal. Echocardiographic findings revealed intra-
cardiac vegetative formations, which have not been reported
before; hence it is quite a rare form of cardiac involvement of
EGPA. We considered adding cyclophosphamide however the
patient’s clinical aspects rapidly improved after intravenous
methyl-prednisolone and surgical treatment.
Conclusion
We experienced a patient with EGPA with cardiac involvement
presenting with non-infectious vegetations. There have been
reports of intra-cardiac thrombus formation in EGPA patients
7
but there are no reports of EGPA-related vegetative formation.
The patient was successfully treated by surgical removal and a
systemic corticosteroid.
References
1.
Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and peri-
arteritis nodosa.
Am J Pathol
1951;
27
(2): 277–301.
2.
Mahr A, Moosig F, Neumann T,
et al
. Eosinophilic granulomatosis with
polyangiitis (Churg-Strauss): evolutions in classification, etiopathogen-
esis, assessment and management.
Curr Opin Rheumatol
2014;
26
(1):
16–23.
3.
Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with poly-
angiitis (Churg-Strauss): state of the art.
Allergy
2013;
68
(3): 261–273.
4.
Szczeklik W, Jakiela B, Adamek D, Musial J. Cutting edge issues in the
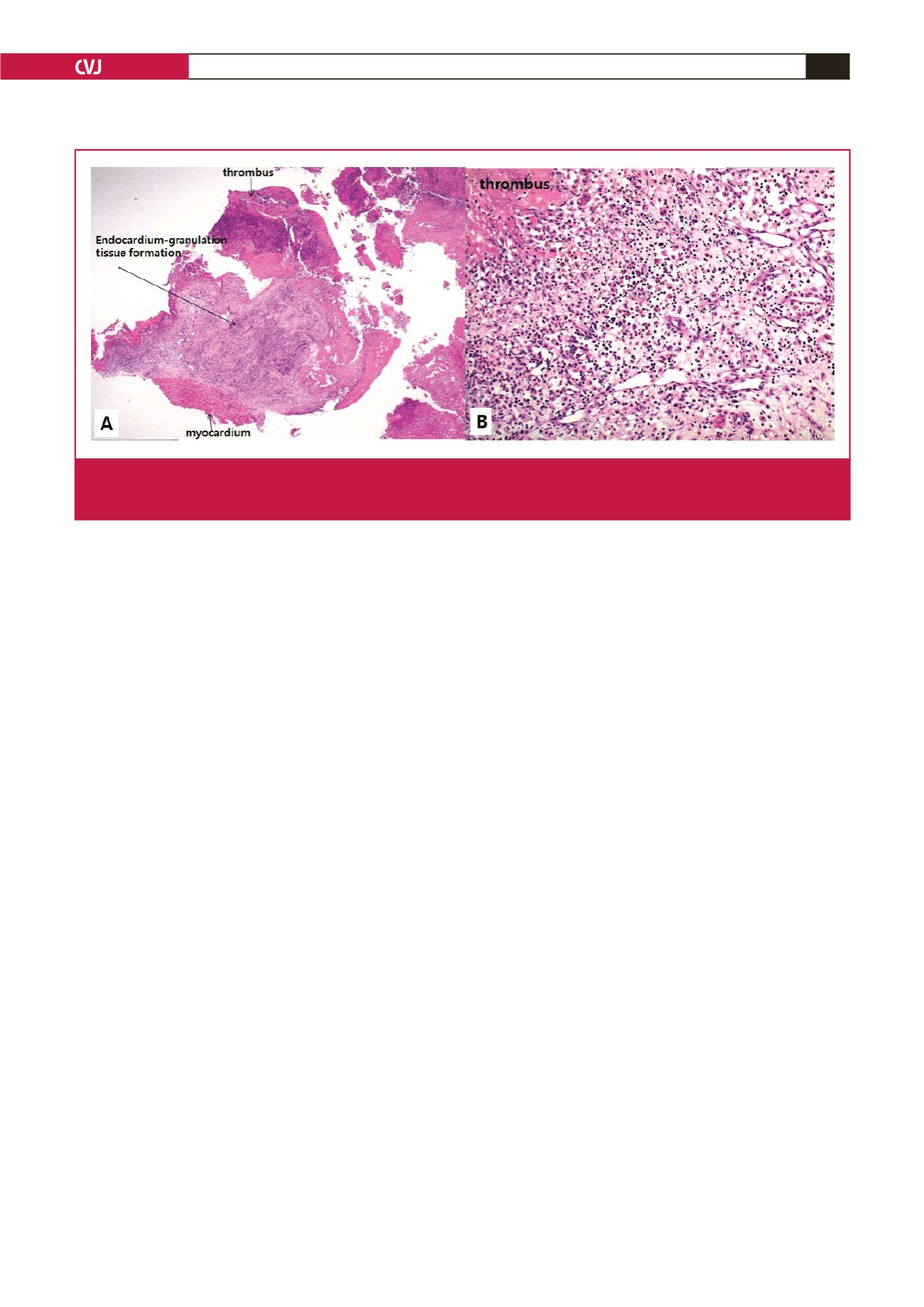
Fig. 3.
A. Microscopic findings revealed marked inflammatory infiltration composed of granulation tissue, eosinophils, lymphoplasma
cells, neutrophils and histiocytes, and thrombus formation (H&E, × 10.25). B. There was eosinophil-rich inflammation in the
endocardium (H&E, × 200).



















