
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 31, No 3, May/June 2020
AFRICA
127
Discussion
To the best of our knowledge, this is the first study that evaluates
cardiac function detected by TDI echocardiography in premature
children after ROP treatment with either anti-VEGF or LPC.
There has been great concern about these new agents due to
their toxic side effects. There are reports of associated systemic
side effects from systemic anti-VEGF agents used in cancer
therapy, such as systemic hypertension, thromboembolism and
LV dysfunction. They are also reported to have several side
effects secondary to intravitreal anti-VEGF therapy, including
systemic hypertension, congestive heart failure, proteinuria,
arterial thromboembolic events, and systemic haemorrhage,
which are linked to cardiovascular toxicities.
16,17
On the other
hand, Scott
et al.
published a commentary about anti-VEGF
agents and the data suggested no difference in the risk of
arteriothrombotic effect or death between anti-VEGF agents.
18
A recent meta-analysis showed 23.6% incidence of all grades
and a 7.9% incidence of high-grade hypertension after systemic
bevacizumab treatment.
19
In the present study, systolic and diastolic blood pressure and
heart rate values in infants were found to be similar to those in the
control group. Development of the blood vessels, vascular growth
and organogenesis are extremely VEGF-dependent, as was
demonstrated with early embryonic lethality caused by a deletion
of the VEGF gene in the signalling pathway.
20
VEGF also has a
role in pathological blood vessel growth (macular degeneration),
tumoural vasculature growth and normal physiological
vasculature growth (menstrual cycle).
21
The endothelium is an
active endocrine organ secreting many cytokines and growth
factors and interacting with cells that affect the function of many
organs such as the heart, kidneys, liver and brain.
After the introduction of intravitreal VEGF treatment,
these agents now have a key role in the treatment of ROP.
Compared to conventional laser therapy, anti-VEGF agents have
some advantages. Although conventional laser therapy led to a
persistent destruction of the peripheral retina, it was shown that
the development of peripheral retinal vessels continued after the
treatment with intravitreal anti-VEGF agents. Due to their ease
of use, these agents are preferred above other treatment options.
Studies demonstrate that by allowing vasculature to develop
further anteriorly and rapidly, intravitreal anti-VGEF treatment
causes less visual loss and fewer refractive errors.
22
In contrast
to the requirement for general anaesthesia in laser therapy,
these agents can be introduced only under topical anaesthesia.
23
They allow the development of the posterior retina and foveal
avascular zone and support the more immediate regression of
ROP than laser treatment.
24,25
Belcik
et al.
reported a significant increase in LV wall thickness
and mass, a decrease in end-diastolic diameter in accordance
with concentric hypertrophy, and they showed a reduction
in thickening fraction and stroke volume over the five-week
anti-VEEF treatment.
26
In our study, we did not observe any
statistically significant differences in LV M-mode measurements,
ejection fraction or fractional shortening between the groups.
MAPSE is another useful evaluation parameter and reduced
MAPSE implies impaired longitudinal function in patients with
various cardiovascular diseases.
27
Our study demonstrated lower
MAPSE values in the IVA and LPC groups, and TAPSE values
were similar. Advanced imaging techniques such as speckle-
tracking methods are needed to prove that reduced MAPSE
values show systolic dysfunction, because MAPSE provides
only LV long-axis systolic performance, whereas other systolic
parameters were in the normal range.
To assess ventricular diastolic function, Doppler data have
an important role to depict distinct patterns of abnormality in
ventricular filling, abnormal relaxation and restrictive filling.
Abnormal relaxation is especially common in disorders producing
myocardial hypertrophy. In such cases, atrioventricular early
filling velocity is decreased and the atrial component of filling
becomes potent.
28
In our study, we observed statistically significant differences
in only both ventricles’ ‘pulsed’ Doppler echocardiography
parameters between the groups, except in E velocity derived
from RV inflow. The IVA and LPC groups had significantly
higher tricuspid E velocity than the control group. It is also
known that during inspiration and apnoea, RV inflow velocities
are significantly higher. Although the E wave represents the
early, rapid-filling period of diastole, higher E-velocity values
of tricuspid inflow were more difficult to interpret than the
impaired relaxation of the right ventricle alone without the E/A
ratio change.
29
Due to such limitations on load conditions, heart rate and
age, which may influence conventional Doppler parameters, TDI
has a major potential in the diagnosis of diastolic ventricular
dysfunction.
30
When diastolic ventricular relaxation is slowed,
prolongation of the isovolumetric relaxation time and a slight
increase in the systolic velocity can be observed.
The MPI is a more specific tissue Doppler parameter for
diastolic dysfunction. In their animal experiment after the anti-
VEGF therapy, using endocardial TDI, Belcik
at al
. reported a
mild decrease in S
′
and E
′
velocities that were not statistically
significant.
26
Various paediatric and adult studies have described
subclinical impairment of systolic function with changes in
MPI, isovolumetric contraction and relaxation time.
31
In our
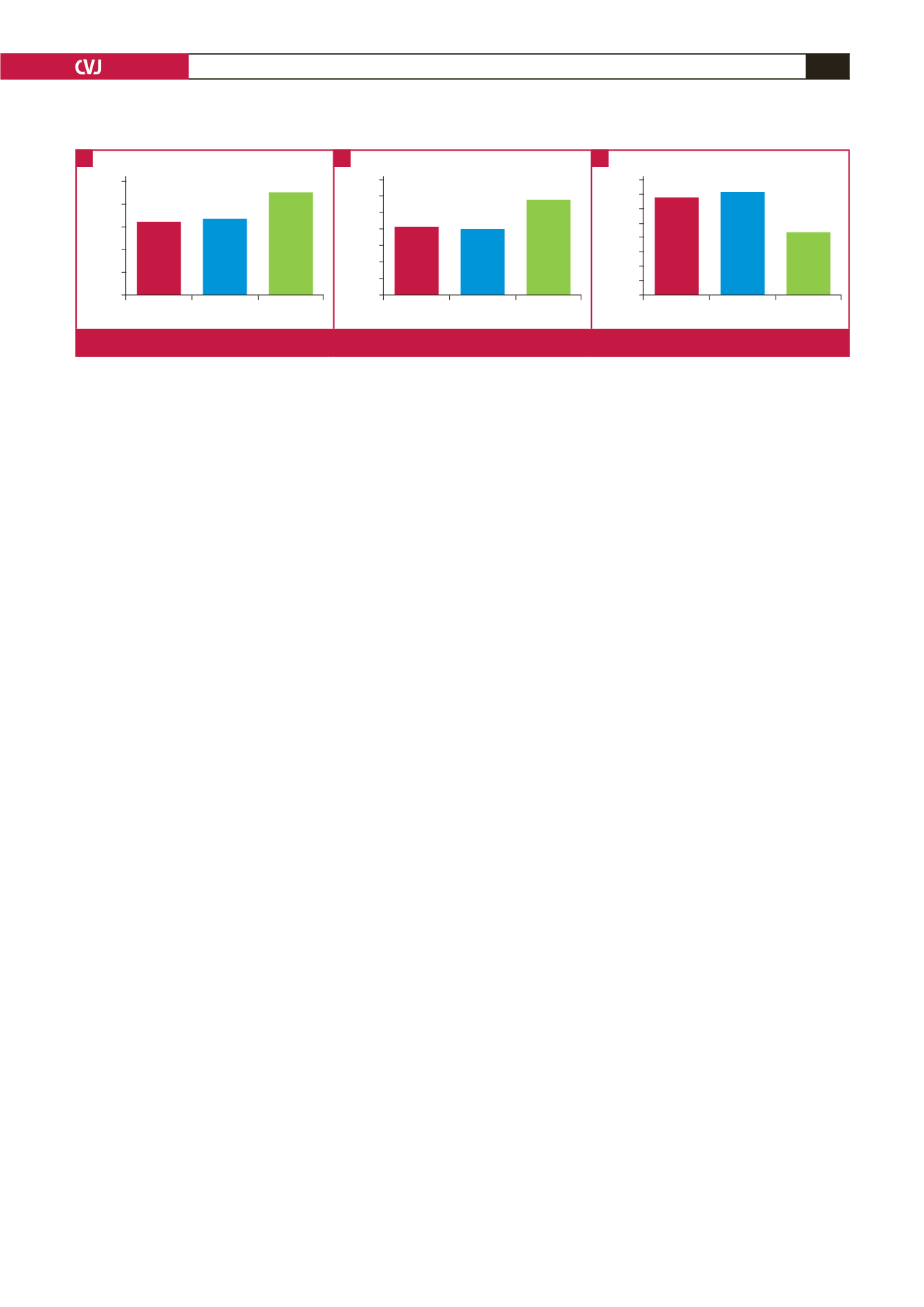
LPC
IVA Control
Aortic strain
25
20
15
10
5
0
16.20
16.70
22.50
LPC
IVA Control
Aortic disensibility
14
12
10
8
6
4
2
0
8.20
8.00
11.60
LPC
IVA Control
Aortic stiffness
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0
3.40
3.60
2.20
Fig. 2.
Comparison of elastic parameters of the ascending aorta of patients and controls.
A
B
C



















