
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 31, No 3, May/June 2020
124
AFRICA
events.
12,13
Therefore, the aim of our study was to compare
changes in aortic elastic parameters, using tissue Doppler and
conventional echocardiographic measurements for both right
and left ventricular systolic and diastolic function after LPC and
IVA therapy in infants with ROP.
Methods
This single-centre, retrospective study was performed by
evaluating the medical records of infants who were treated for
ROP in a tertiary centre for screening and treatment of ROP. The
study was carried out between October 2016 and February 2017.
The institutional reviewboard at AdanaNumune Training and
Research Hospital approved the study. Informed written consent
was obtained from all parents or guardians. All procedures
performed were in accordance with the ethical standards of the
institutional and/or national research committee and with the
1964 Helsinki Declaration and its later amendments.
During this period, premature infants who completed the
corrected age of one year were evaluated using echocardiography
for routine cardiac control. Decision about the treatment option
for ROP was made as reported by the indications in the Early
Treatment for ROP (ETROP) study.
3
According to the study,
infants with type 1 pre-threshold ROP, threshold ROP or
aggressive posterior ROP were selected. If infants had treatment-
requiring ROP in the posterior zone (zone I and/or zone II),
anti-VEGF treatment was recommended for them because
laser treatment has low efficacy in posterior disease, along with
decreased visual field and high refractive outcomes.
All parents were informed about the treatment effects and
systemic concerns of the IVA. They were also informed about
LPC treatment regarding its lower efficacy in posterior ROP,
and possible side effects such as preventing peripheral retinal
vascularisation. The parents were then left with the decision of
whether to treat with LPC or IVA. Patients who received laser
or aflibercept treatment as monotherapy and primary treatment
were included in the study.
Patients who were treated at a different centre, who were
administered another treatment option (cryotherapy, surgery
and other anti-VEGF agents) or combined therapy (infants who
received additional treatment after primary treatment) for ROP
and who could not be followed regularly were excluded from the
study. Infants with stage 4 and 5 ROP or infants who underwent
vitreoretinal surgery were also excluded, as were those who had
systemic or ocular disease such as congenital cataract, glaucoma
or other ocular anomalies. Further exclusions were infants with
congenital heart, lung or other systemic disease and dysrhythmia.
Twenty age- and gender-matched patients were selected from
among the patients who were referred for evaluation of an ROP
screening and who did not receive any treatment. They were
found to have normal intra-cardiac structural anatomy and
function.
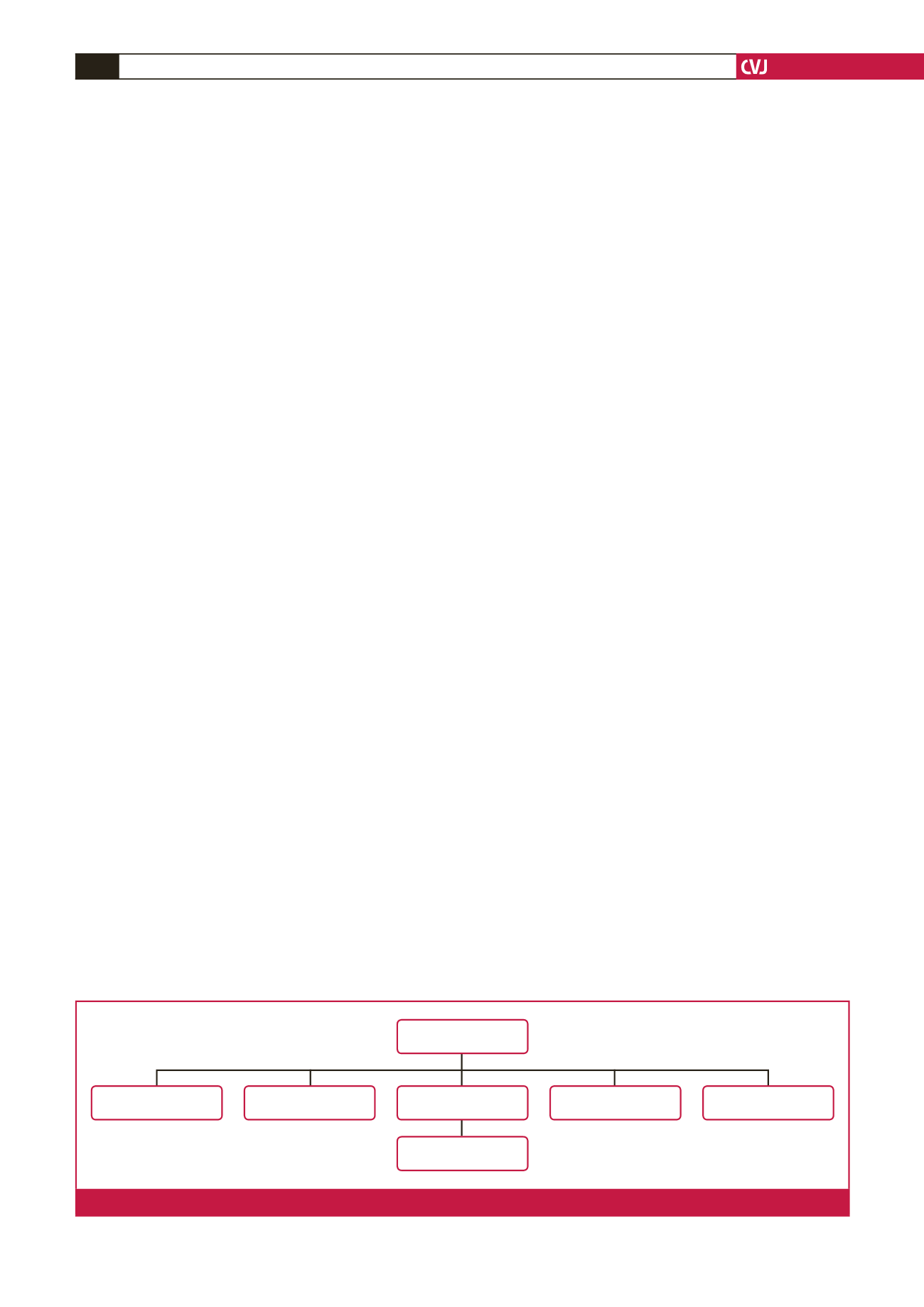
In this period, 67 medical records were reviewed. Among
them, four patients were treated with combined therapy, six
received other anti-VEGF agents, two were treated at a different
centre, three had ocular or systemic anomaly, and one patient
had stage 4 ROP. These 16 patients were excluded from the study
(Fig. 1).
Thirty-one infants with a history of prematurity who
underwent treatment for ROP were selected for the patient
groups, and 20 infants diagnosed with ROP but who did not need
any treatment were selected as the control group. Infants with
similar demographic features (age, gender, gestational age, birth
weight) were involved in this study.
A total of 31 premature infants with ROP and 20 premature
infantswithoutROPwere included in this study. Theyweredivided
into three groups: the LPC group included 15 infants (mean age:
17 ± 4.4 months) who received diode laser photocoagulation;
and the IVA group included 16 infants (mean age: 14.4 ± 4.9
months) who received only a single dose of intravitreal injection
of aflibercept (1 mg/0.025 ml) as the primary treatment for ROP;
and 20 infants constituted the control group (mean age: 14.5 ±
2.8 months).
Height, weight, birth weight, gestational age and heart rate
of the infants were recorded in both patient groups. Ten minutes
after calming down in the room and while the infants were held in
their parents’ laps, a validated oscillometric device (Omron HEM
907; Omron Healthcare, Kyoto, Japan) was used to measure
systolic and diastolic blood pressure in the right arm with an
appropriate cuff size covering two-thirds of the upper arm.
Laser ablations were performed with an 810-nm diode laser
(IRIDEX; Oculight SL, Mountain View, CA, USA) using a
28-day condensing lens. The laser settings were arranged to a
power ranging between 150 and 250 mW with a duration of
200 m/s and an interval of 200 s, so that a moderately white
laser burn could be achieved. All patients received intravitreal
aflibercept (Eylea
®
, Regeneron Pharmaceuticals Inc, Tarrytown,
New York, USA) 1 mg/0.025 ml in the operating room
under sterile conditions with topical anaesthesia, using 0.5%
proparacaine hydrochloride (Alcaine; SA Alcon-Couvreur NV,
Puurs, Belgium) and ketamine sedation. All the treatments were
performed by the same specialist (EAS).
47 infants
4 combined therapy
2 other centres
6 other anti-VEGF
agents
31 evaluated and
screened
3 ocular and systemic
anomaly
1 stage 4 ROP
Fig. 1.
Flow chart of the study population.



















