CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 4, May 2013
108
AFRICA
procedure. All patients had been pre-treated on the day of the
procedure with aspirin 300 mg and clopidogrel 600 mg.
Ten procedures were done at Vergelegen Medi-Clinic,
Somerset West. The first seven were with proctor guidance.
One was done at Groote Schuur Hospital, Cape Town and one at
Vincent Pallotti Hospital, Cape Town. All procedures were done
under general anaesthetic, as transoesophageal echocardiogram
(TOE) guidance is required during the procedure. Trans-septal
puncture was done via the right femoral vein in the usual manner
using TOE guidance. Following trans-septal puncture, 5 000
units of intravenous heparin was given and ACT was maintained
>
250 s for the procedure.
An angiogram was then taken of the left atrial appendage
to size the left atrial appendage orifice. A suitably sized device
was then selected and placed in the appropriate position using
TOE and angiographic guidance. All patients had a transthoracic
echocardiogram after the procedure and the next day to exclude
a pericardial effusion or device shift.
Patients were discharged home the following day on aspirin
82–150 mg daily indefinately and clopidogrel 75 mg daily for a
month. All patients were seen at one month post procedure with
a transthoracic echocardiogram, and six-monthly thereafter.
Results
There were no procedure-related complications and 100%
implantation success was achieved. In one case the initial device
chosen was too small and a second larger device was chosen
(Table 2, procedure characteristics). The average device size was
25.8 mm. All patients were disharged home the following day.
There were no serious safety events, particularly pericardial
effussion, device embolisation or procedure-related stroke
(Table 3). No patients had pericardial effusions seen on echo
immediately after the procedure and on discharge the following
day.
Clopidogrel was stopped in all patients at one month, except
one patient who stopped the clopidogrel after one week due to
recurrent epistaxis (Patient 4). On follow up varying between
three and 20 months, no cardiac embolic events had been
recorded in any patients. All devices are well seated and there
have been no device-related complications.
Discussion
Occluding the LAA in patients with AF and thereby preventing
the vast majority of intracardiac thrombus formation is a highly
attractive concept, especially for those patients who are unable
to take any form of oral anticoagulant therapy. The patients,
however, must be made aware that exclusion of the LAA, similar
to when using warfarin, does not absolutely exclude risk of
future strokes.
The Protect AF study,
16
using the Watchman device,
randomised 542 AF patients, 2:1 between the device versus
warfarin. This study showed the device was non-inferior to
warfarin in terms of stroke prophylaxis with a trend towards
superiority. In the successfully treated population (device
deployed and warfarin stopped) the primary efficacy event rate
(all stroke) in the intervention group who discontinued warfarin
was 1.9 per 100 patient years compared with 4.6 per 100 patient
years in the control group who received warfarin (RR
=
0.40).
Primary safety events occurred at a higher rate in the
intervention group than in the control group (7.4 per 100
patient years vs 4.4 per 100 patient years; RR
=
1.69). The most
frequent primary safety event in the intervention group was
serious pericardial effusion (requiring drainage), which occurred
in 4.8% of patients. No patients with pericardial effusion died.
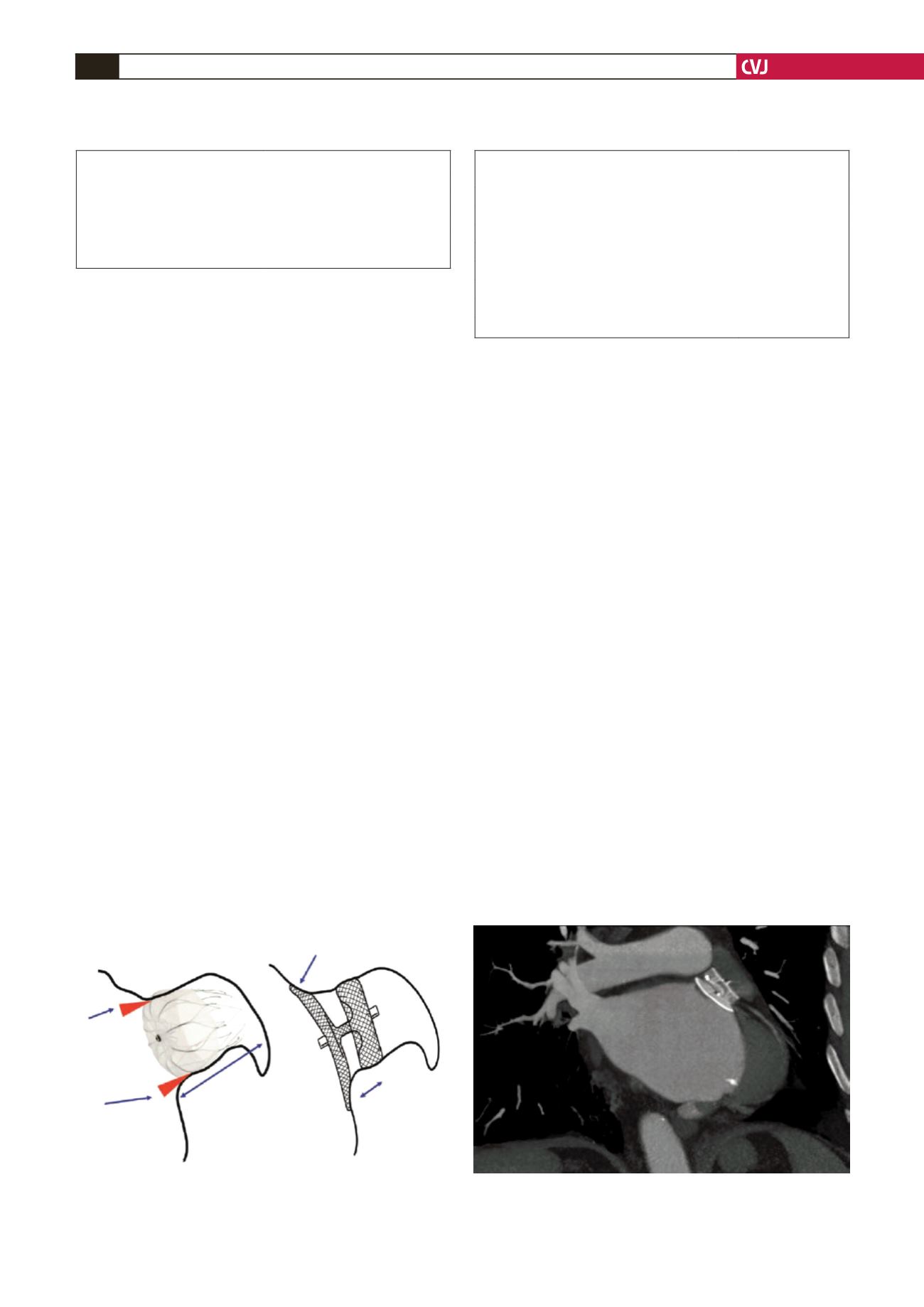
Fig. 2. Amplatzer cardiac plug seen on CT scan occluding
the left atrial appendage orifice 12 months post deploy-
ment.
TABLE 3. COMPLICATIONS – ACUTEAND ON FOLLOW UP
(
n
=
12)
Cardiac tamponade
0
Pericardial effusion
0
Device embolisation
0
Procedure-related stroke
0
Subsequent stroke/embolic event off warfarin
0
Access site complications
0
Death, all cause
0
Duration of follow up (mean)
12.8 months (2–20)
TABLE 2. PROCEDURE CHARACTERISTICS (
n
=
12)
Implantation success
12
Residual leak
1
Device size
25.8 mm (22–30)
Fluoroscopy time
23.14 min (14.9–35.4)
Hospital stay duration
1 day in all patients
Fig. 1. Schematic diagrams illustrating the difference
between ACP and Watchman devices (with permission
John Wiley and Sons
18
).
Watchman device
Amplatzer cardiac plug
Potential
source of
leakage
between
device and
LAA orifice
Deep LAA anatomy
is required for
Watchman device
Disc to seal LAA orifice
ACP implanta-
tion is possible
in patient with
shallow LAA
because of short
device length


