CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 7, August 2013
258
AFRICA
had more subjects with a history of smoking than the vitamins
C
+
E and control groups, but this was not important because all
smokers refrained from smoking for at least 48 hours before the
study to prevent any significant effects of smoking.
Several large epidemiological studies have suggested that
dietary intake of vitamin C is inversely associated with the risk of
ischaemic heart disease.
11
In other studies, however, vasodilation
was observed to increase over a period of only two hours.
11,12
We
repeated the measurements after two hours of administration
because Levin
et al
. showed that plasma ascorbic acid levels
reached a plateau after two hours and remained elevated five
hours after ingestion.
13
On the other hand, Westhuyzen
et al
.
showed that
α
-tocopherol concentrations after oral intake had
reached the same levels by the second hour and stayed at that
level for almost five hours.
14
We therefore did not measure the
blood levels of vitamins C and E.
Drossos
et al
. showed that vitamin C, like vitamin E, has a
potent vasodilating effect on the radial artery.
15
They examined
the dilation of the lumen surface and colour Doppler images of
the non-dominant radial artery just before and two hours after
oral vitamin C administration. The results provided evidence
that vitamin C was a potent vasodilator in healthy subjects,
particularly in smokers. In addition, it was a superior acute
vasodilating agent
in vivo
compared with diltiazem in ischaemic
patients awaiting cardiac surgery.
15
In our study, we used the same method to measure radial
artery vasodilation. We took measurements at the time of cuff
deflation and 60 seconds later to observe the effect of vitamin C
on the endothelium.
Excessive vascular oxidative stress has been linked to
impaired endothelium-dependent arterial relaxation in coronary
artery disease. Keaney
et al
. showed in their study the beneficial
effects of vitamin E on endothelial function.
16
Vitamins C and E may favourably influence cardiovascular
risk, but there are several important differences between these
naturally occurring antioxidants. Vitamin C is water soluble, and
is present in most body fluids. However, vitamin E is a lipid-
soluble antioxidant.
The primary antioxidant mechanisms of these antioxidants
are also distinct. The important antioxidant properties of vitamin
C are its abilities to scavenge superoxide anions and to preserve
reduced intracellular glutathione concentrations. Also, vitamin
C is required for the regeneration of vitamin E.
17
Vitamin C
may thus prevent low-density lipoprotein (LDL) oxidation,
either through the recycling of vitamin E or by scavenging free
radicals directly.
18
We therefore observed the beneficial effects of
vitamins C and E on endothelial function in our study.
In a recent double-blind trial, Brown
et al
. studied simvastatin–
niacin and antioxidant vitamin therapy, alone and together, for
cardiovascular protection in patients with coronary disease and
low plasma levels of high-density lipoprotein (HDL) cholesterol.
19
The baseline levels of LDL cholesterol and triglycerides decreased
when antioxidant vitamins were added to the simvastatin–niacin
regimen. The HDL level increased by 18% in those treated with
simvastatin–niacin and antioxidant vitamins. With simvastatin–
niacin and antioxidant vitamin therapy, the levels of HDL2 and
apolipoprotein A-I [Lp(A-I)] increased by 81%. The resistance of
LDL to oxidation increased by 35%.
In an another study, Behrendt
et al
. showed that vitamin
C and E combinations reduced cardiac transplant-associated
arteriosclerosis in patients with normal or abnormal endothelial
function. The magnitude of benefit was larger in patients with
endothelial dysfunction.
20
Conclusion
This study demonstrated that oral administration of the
antioxidants vitamins C and E in physiological doses may
enhance endothelium-dependent vasodilatation in the radial
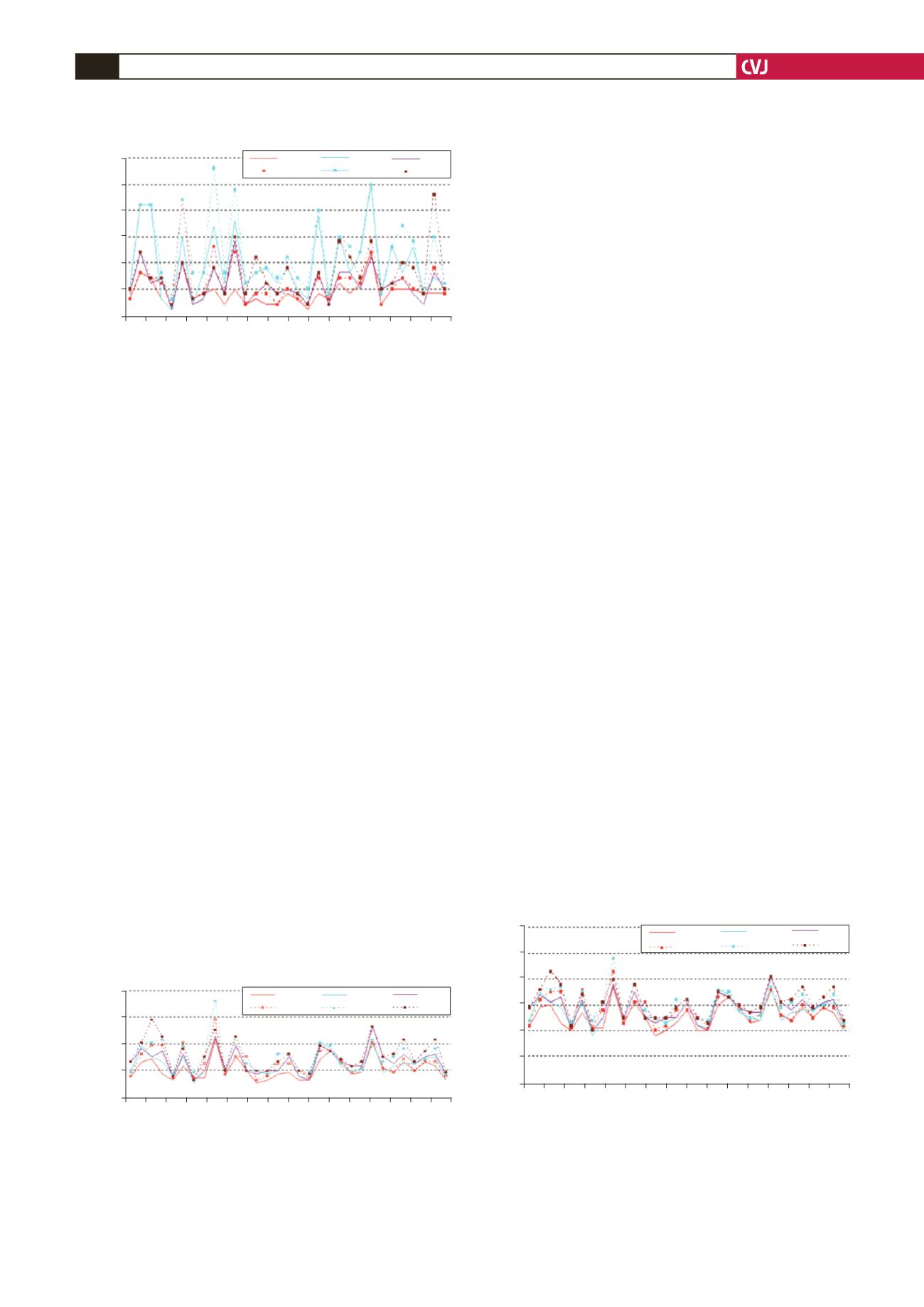
Fig. 4. Flow volume measurements on the radial artery
before and after oral vitamins C
+
E administration. FV1,
FV4, baseline flow volume before and after vitamin C
+
E,
respectively; FV2, FV5, flow volume at the moment of cuff
deflation before and after vitamins C
+
E, respectively;
FV3, FV6, flow volume 60 seconds after cuff deflation
before and after vitamin C, respectively.
0.3
0.25
0.2
0.15
0.1
0.05
0
1
3 5 7 9 11 13 15 17 19 21 23 25 27 29 31
Patients
Flow volume (l/min)
FV1
FV4
FV2
FV5
FV3
FV6
Fig. 5. Radial artery area measurements before and after
oral vitamins C
+
E administration. A1, A4, baseline area
before and after vitamins C
+
E, respectively; A2, A5, area
at the moment of cuff deflation before and after vitamins
C
+
E, respectively; A3, A6, area 60 seconds after cuff
deflation before and after vitamins C
+
E, respectively.
20
15
10
5
0
1
3 5 7 9 11 13 15 17 19 21 23 25 27 29 31
Patients
Radial artery area (mm
2
)
A1
A4
A2
A5
A3
A6
Fig. 6. Radial artery diameter before and after oral vita-
mins C
+
E administration. D1, D4, baseline diameter
before and after vitamins C
+
E, respectively; D2, D5,
diameter at the moment of cuff deflation before and
after vitamins C
+
E, respectively; D3, D6, diameter 60
seconds after cuff deflation before and after vitamins C
+
E, respectively.
6
5
4
3
2
1
0
1
3 5 7 9 11 13 15 17 19 21 23 25 27 29 31
Patients
Radial artery diameter (mm)
D1
D4
D2
D5
D3
D6


