
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 4, July/August 2015
182
AFRICA
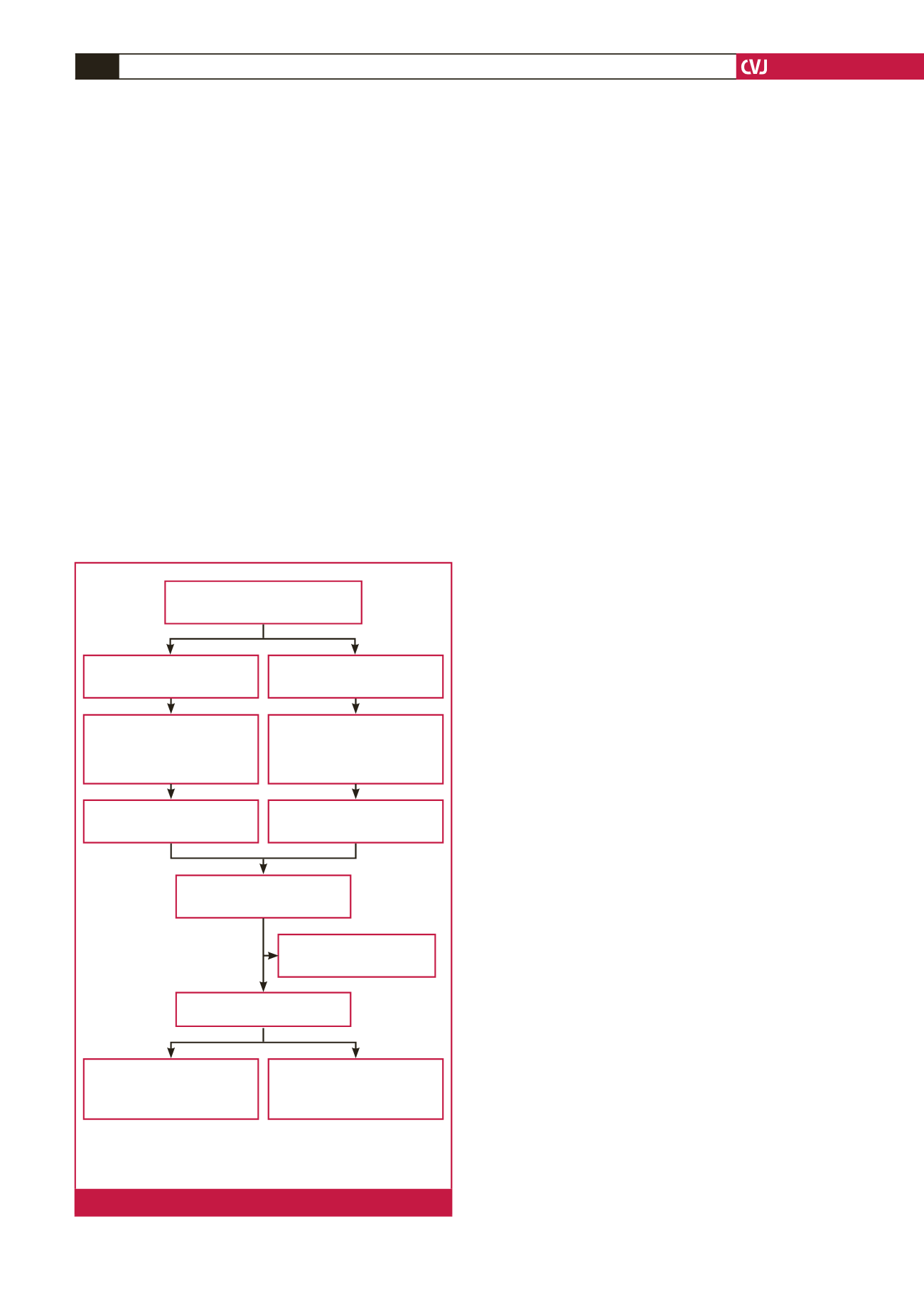
(3.8%) re-used devices implanted, as shown in Fig. 1. Patients
with re-used devices (cases) were then matched by age, gender
and date of implantation on a 1:1 basis to patients with new
devices (controls). In the pacemaker group, cases and controls
were matched to the same month of implantation, and for the
ICD group, to the same year of implantation.
Devices for re-use were obtained from cadaveric donors. They
were inspected for external damage and tested for remaining battery
life. Devices with less than two years of battery life remaining and/
or with external evidence of damage were not re-used. Only devices
with two or more years of battery life remaining with no evidence
of external damage were eligible for re-use.
The eligible devices were sterilised by immersion in biozyme
for 24 hours, followed by peroxide for a further 24 hours and
then orthozyme for another 24 hours. After the three days of
chemical treatment, the devices were dried out using pressurised
air and subsequently subjected to gas sterilisation. In the gas
sterilisation unit, they were put in a machine with ethylene oxide
for 4.5 hours and irradiated for two cycles of 30 minutes, three
days apart.
After device sterilisation, all devices were checked by a cardiac
technologist in the department for any visual defects and for
device longevity, and were tested to determine whether they
were functioning appropriately for re-use. Device manufacturer’s
personnel were not involved in this process.
A cardiac technologist or cardiology register in training was
present at every implant procedure. Standard measurements
were obtained during the implant after lead positioning (capture
thresholds, battery life, sensitivities and lead impedances) and
again prior to discharge.
Re-used pacemakers were implanted mainly in elderly
patients with multiple co-morbidities such as advanced cancer
(on treatment or in remission), cerebrovascular accident (CVA),
advanced chronic obstructive pulmonary disease (COPD),
dementia and/or a poor baseline level of functioning (mostly
bed bound) who were expected to have a significantly reduced
life expectancy. Re-used ICDs were implanted in patients who
met the secondary prevention criteria for sudden death, and
co-morbidity was not a factor in determining who received a
re-used ICD.
The inherent difference between patients who received re-used
pacemakers compared to those who had new pacemakers led
us not to compare the outcome of patients in the two groups.
The units of analysis were the devices themselves. Every patient
provided a written informed consent for implantation of the
device.
The devices were implanted by a cardiac electrophysiologist,
cardiologist or a cardiology senior registrar. Prior to
implantation, patients received 1 g of intravenous infusion of
cefazolin as prophylaxis. Patients were discharged from hospital
the following day provided there were no complications and were
followed up in the pacemaker clinic at three months and yearly
thereafter. Patients with ICDs were followed up more frequently
at three- to four-monthly intervals.
Outcomes
The outcomes of interest were procedure-related infection, device
malfunction, early battery depletion, and device explantation for
infection, malfunction and/or battery depletion. The definitions
of the outcomes are as follows.
•
Procedure-related infection: infections were classified into four
types:
23
(1) right-sided endocarditis with lead involvement; (2)
sepsis with evidence of involvement of the lead and implan-
tation pocket; (3) involvement of the pacemaker implanta-
tion pocket; and (4) involvement of the lead or generator.
Infections were considered early if the onset of illness was
within the first month of implantation, and late if the onset
of illness was after the first month to a year after implanta-
tion.
23
Infections that occurred after a year of implantation
were considered not to be related to the procedure.
23
•
Device malfunction was defined as failure of the device to
accomplish the desired role, e.g. in the case of an ICD, not
able to sense ventricular tachycardia/fibrillation and deliver
appropriate treatment. In the case of a pacemaker, device
malfunction was defined as inability to sense or pace when
required.
•
Early battery depletion was defined as battery depletion
within six years of implantation for new devices. For re-used
devices, early battery depletion was defined as battery deple-
tion within one to two years of implantation for those with
two to four years of battery life remaining, and within two
Devices implanted 2003–2013
(
n
=
1721)
Pacemakers,
n
(%) – 1 587 (92.2)
ICDs,
n
(%) – 134 (7.8)
First implants,
n
(%) – 114 (85.1)
Generator change
n
(%)
– 20 (14.9)
First implants,
n
(%) – 1257 (79.2)
Generator change
n
(%)
– 330 (20.8)
Re-used ICDs,
n
(%) – 12 (9)
Re-used pacemakers,
n
(%) – 54 (3.4)
Re-used devices,
n
(%) – 66 (3.8)
Excluded,
n
(%) – 3 (4.5)
Missing data
Analysed (
n
=
126)
Pacemakers,
n
(%) – 102 (81)
Re-used,
n
(%) – 51 (50)
New,
n
(%) – 51 (50)
ICDs,
n
(%) – 24 (19)
Re-used,
n
(%) – 12 (50)
New,
n
(%) – 12 (50)
ICDs
=
implantable cardioverter defibrillators
n
=
number
(%)
=
percentage
Fig 1.
Outline to assess eligibility for enrolment.

















