
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 27, No 1, January/February 2016
8
AFRICA
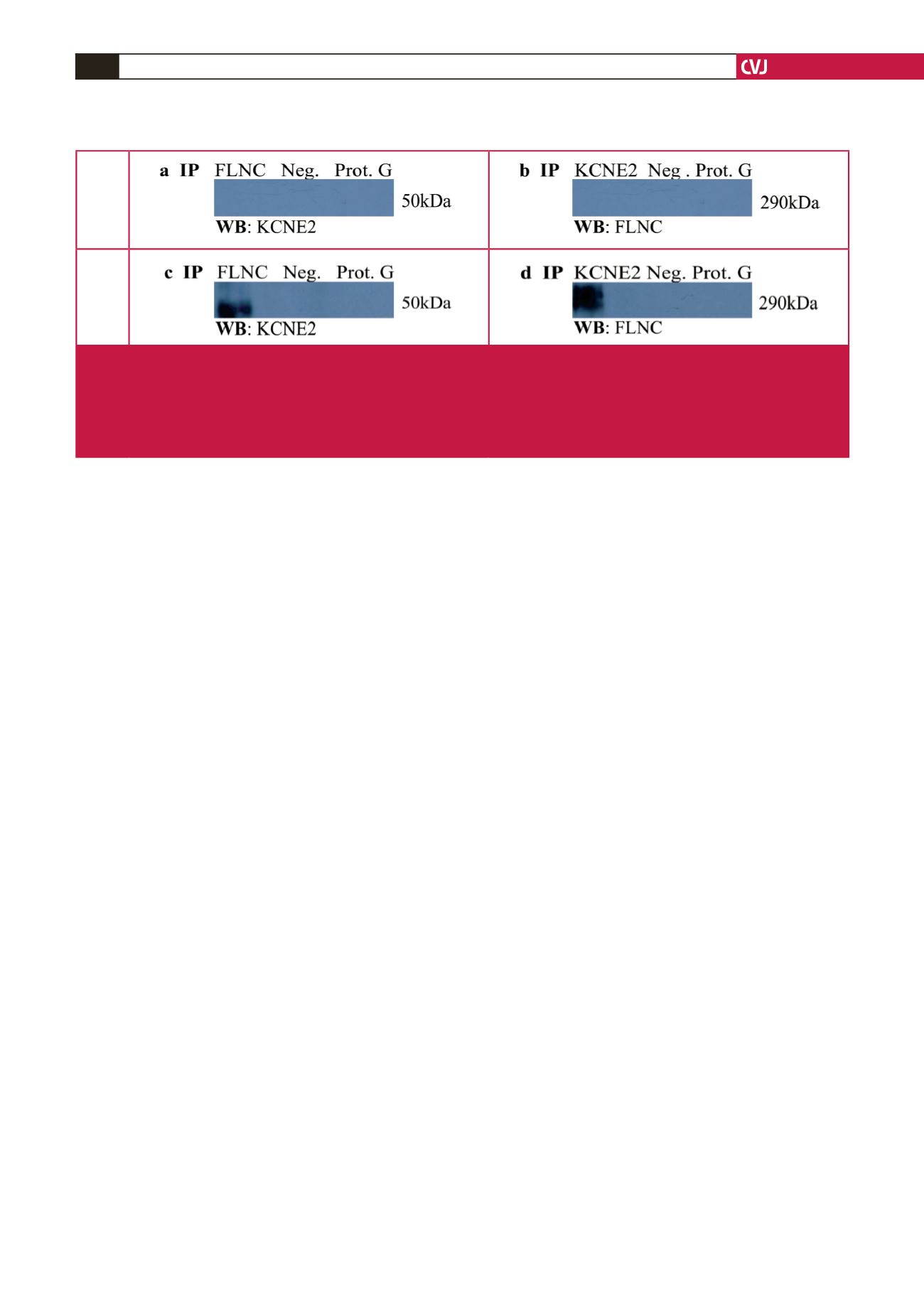
Under normoxic conditions, reciprocal Co-IP experiments failed
to show any physical interaction between KCNE2 and FLNC (Fig.
2a, b). However, during hypoxia an interaction between KCNE2
and FLNC was observed (Fig. 2c, d). These findings suggest that
the induction of stress is essential to the interaction and one could
speculate that hypoxia-induced conformational changes of FLNC
are necessary for the KCNE2–FLNC interaction.
Discussion
This study identified a novel protein–protein interaction between
the cytoplasmic C-terminal domain of KCNE2 and FLNC
during conditions of acute hypoxia. To date, the intracellular
C-terminal domain residues of KCNE2 have been implicated in
modulating HERG current density,
13,41
current deactivation rates,
41
and phosphorylation-dependant channel degradation.
19
However,
studies elaborating on specific regulatory roles for this domain
remain scarce, highlighting the importance of the current findings.
The interactor identified in this study, FLNC, is located in
the cytoplasm at the Z-line of the sarcomere and functions
in the cytoskeleton, where it is involved in crosslinking actin
filaments into networks and anchoring membrane proteins.
32,42
This filamin and its main paralogs, FLNA and FLNB, act as
scaffolding proteins and have been implicated in a number of
cellular stress responses,
32-34,43-46
including several hypoxia-related
effects.
33,34,45,46
FLNC specifically, is predominantly expressed in
muscle tissue and is associated with cardiac abnormalities such
as desminopathy, characterised by muscle weakness, conduction
blocks, arrhythmias and chronic heart failure, frequently resulting
in sudden cardiac death.
35,36
Filamins also play an important part in cell signalling
by disrupting existing interactions or by the introduction of
novel interactions.
32,37,38,47
Interestingly, there are several reports
detailing interactions of filamin family members with ion
channel subunits.
48-50
Particularly noteworthy is a previously
descibed association in neuronal tissue between FLNC and
the potassium voltage-gated channel subfamily D member 2
(KCND2),
48
the
α
-subunit of the Kv4.2 channel. That study
proposed that FLNC mediates the direct link between KCND2
and the actin cytoskeleton and showed that this interaction is
essential for the generation of appropriate current densities.
48
Both neuronal and cardiac tissue contain voltage-gated ion
channels responsible for controlling the excitability of neurons
and cardiomyocytes. These channels allow for communication
between cells in these tissues.
51-53
Furthermore, a KCNE2–
KCND2 interaction has been described, implicating KCNE2 in
the regulation of the rapidly inactivating KCND2
α
-subunit.
54,55
A common theme in the observation of the ion channel
interactions with filamin is the ability of filamin to influence
membrane localisation.
48-50
For FLNC, this process has also been
shown to involve other actin-binding and auxiliary ion channel
proteins.
56
In the present study, the C-terminal of FLNC, specifically
amino acids 2637–2725 (GenBank: NP_001449.3), bound to
the cytoplasmic C-terminal domain of KCNE2, exclusively
during conditions of hypoxic stress. This finding is consistent
with a number of other studies, indicating that the C-terminal
region of filamins is involved in protein interactions.
57
The
FLNC amino acid residues defined to interact with KCNE2
in this investigation correspond to a domain that is responsible
for protein dimer formation and is important for actin filament
bundling and cross-linking activities.
58,59
The introduction of hypoxic stress is known to have profound
effects on the cell.
60
These include the disruption of ionic
homeostasis, mitochondrial dysfunction resulting in impaired
ATP production, induction of cell death by apoptosis or necrosis,
and the generation of reactive oxygen species (ROS).
61
Excess
ROS leads to cardiac cell damage and post-ischaemic contractile
dysfunction by attacking virtually all cellular components.
62
This results in the degradation of intracellular proteins, rupture
of cellular membranes (including the sarcolemma), as well as
intracellular calcium ion overload,
63
however, it has been show
that cells remain viable even after extended periods of hypoxic
stress.
64
In addition to this, the actin cytoskeleton is also severely
compromised and may therefore be a driving force for novel
interactions. Additionally, Kesner
et al
. indicated that stress-
induced conformational changes in filamins could have a direct
effect on existing interactions or may influence the presence of
novel interactions.
47
Co-localisation analysis revealed that KCNE2 and FLNC
co-localise under both normoxic and hypoxic conditions.
However, no physical interaction could be confirmed between
Normoxic
Hypoxic
Fig. 2.
Western blots of Co-IP of KCNE2 and putative interactor FLNC in differentiated H9C2 cardiomyocytes. Reciprocal Co-IP
reactions were performed for each interaction. (a–b) Co-IP under normoxic conditions. (c–d) Co-IP under hypoxic condi-
tions. FLNC: filamin C; IP: immunoprecipitate; KCNE2: potassium voltage-gated ion-channel subfamily E member 2; kDa:
kilo Dalton; Neg: negative control; Prot G: protein G agarose control; WB: Western blot. Note: The Western blot revealed a
larger-than-expected predicted molecular weight band for KCNE2 (50 kD versus 14–20 kDa) (c). This is likely due to previ-
ously described protein modifications and/or protein interactions of KCNE2.
19,77-80

















