
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 27, No 2, March/April 2016
76
AFRICA
Conclusion
The exact aetiology of PE remains elusive but much of the
pathophysiology has been explained. The current theory is one
of balance between angiogenic and anti-angiogenic factors.
Measurement of circulatory angiogenic and anti-angiogenic
proteins as biomarkers could possibly indicate placental
dysfunction and differentiate PE from other disorders, such as
gestational hypertension and chronic glomerulonephritis. In
addition, biomarkers such as those stated above are reproducible,
linked to the disease, and above all, are easy to interpret.
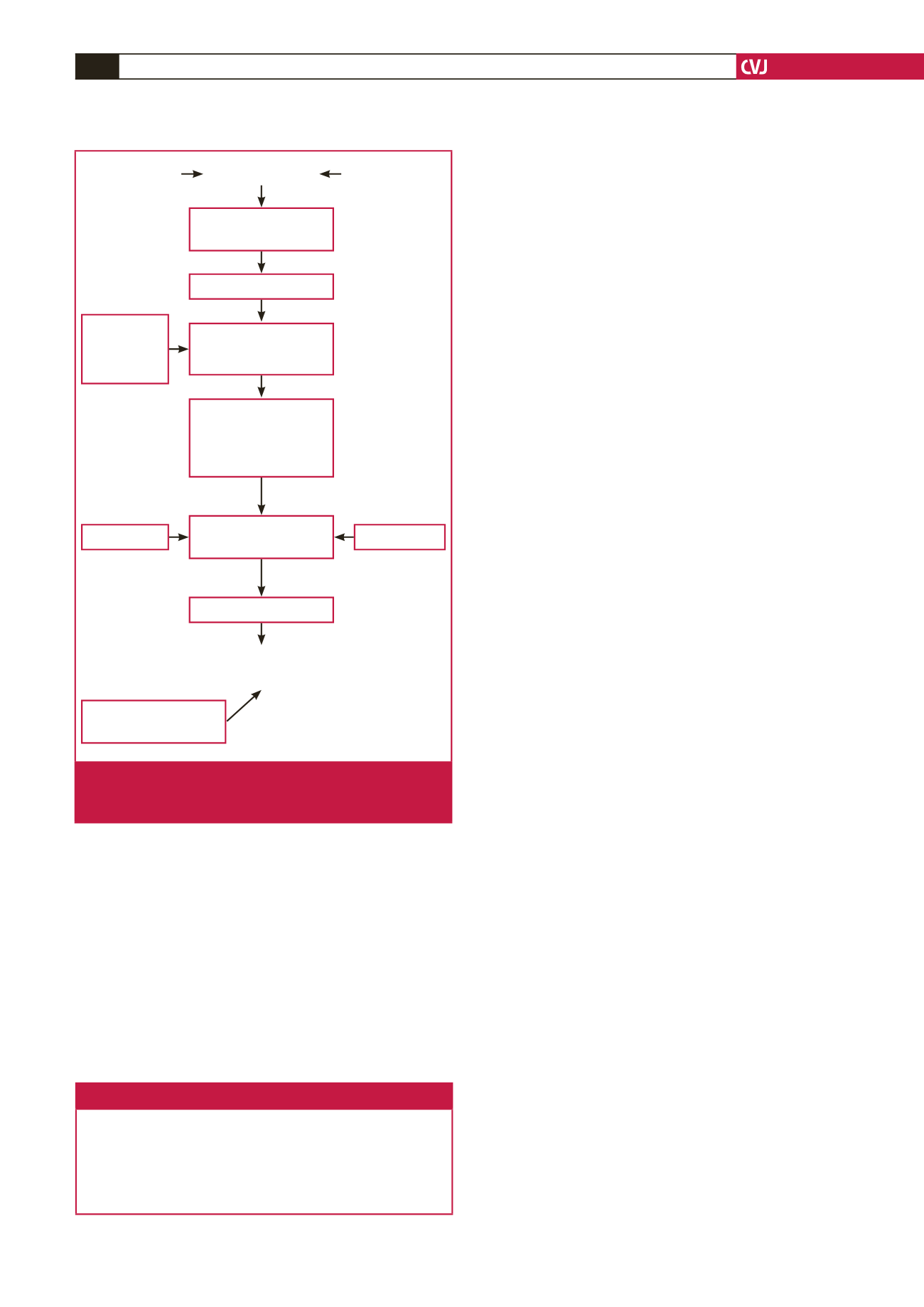
See Fig. 1 for some aspects of the pathophysiology of
pre-eclampsia.
Key messages
• The exact aetiology of pre-eclampsia remains elusive.
• Much of the pathophysiology has been explained.
• Treatment remains empirical and cure is dependent on stabilisa-
tion of high blood pressure and other specific organ complications,
followed by delivery of the foetus and placenta.
References
1.
American College of Obstetricians and Gynecologists. Task force on
hypertension in pregancy. Hypertension in Pregnancy 2013. http://www.
acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Hypertension_in_Pregnancy (accessed 2 June 2014).
2.
O’Tierney-Ginn PF, Lash GE. Beyond pregnancy: modulation of
trophoblast invasion and its consequences for fetal growth and long-
term children’s health.
J Reprod Immunol
2014;
104–105
: 37–42.
3.
Romero R, Chaiworapongsa T. Preeclampsia: a link between tropho-
blast dysregulation and an antiangiogenic state.
J Clin Invest
2013;
123
(7): 2775–2777.
4.
Staff AC, Benton SJ, von Dadelszen P,
et al
. Redefining preeclampsia
using placenta-derived biomarkers.
Hypertension
2013;
61
(5): 932–942.
5.
von Dadelszen P, Magee LA, Roberts JM. Subclassification of preec-
lampsia.
Hypertens Pregnancy
2003;
22
(2): 143–148.
6.
Huppertz B. Placental origins of preeclampsia: challenging the current
hypothesis.
Hypertension
2008;
51
(4): 970–975.
7.
Sohlberg S, Mulic-Lutvica A, Lindgren P, Ortiz-Nieto F, Wikstrom AK,
Wikstrom J. Placental perfusion in normal pregnancy and early and
late preeclampsia: a magnetic resonance imaging study.
Placenta
2014;
35
(3): 202–206.
8.
Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late pre-
eclampsia: two different maternal hemodynamic states in the latent
phase of the disease.
Hypertension
2008;
52
(5): 873–880.
9.
Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta
function and the prediction of preeclampsia and intrauterine growth
restriction.
Prenatal Diagn
2010;
30
(4): 293–308.
10. Verdonk K, Visser W, Van Den Meiracker AH, Danser AH. The renin-
angiotensin-aldosterone system in pre-eclampsia: the delicate balance
between good and bad.
Clin Sci (Lond)
2014;
126
(8): 537–544.
11. Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-
mediated vasodilation in human pregnancy.
Am J Physiol
1997;
272
(2
Pt 2): H748–752.
12. Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal
plasma volume and hormonal changes in women with preeclampsia or
fetal growth restriction.
Hypertension
2006;
47
(2): 203–208.
13. Zhou Y, McMaster M, Woo K,
et al.
Vascular endothelial growth
factor ligands and receptors that regulate human cytotrophoblast
survival are dysregulated in severe preeclampsia and hemolysis, elevated
liver enzymes, and low platelets syndrome.
Am J Pathol
2002;
160
(4):
1405–1423.
14. Valenzuela FJ, Perez-Sepulveda A, Torres MJ, Correa P, Repetto
GM, Illanes SE. Pathogenesis of preeclampsia: the genetic compo-
nent.
J Pregnancy
2012; Article ID: 632732: 8 pages.
http://dx.doi.org/10.1155/2012/632732.
15. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great
Obstetrical Syndromes” are associated with disorders of deep placenta-
tion.
Am J Obstet Gynecol
2011;
204
(3): 193–201.
16. Foidart JM, Schaaps JP, Chantraine F, Munaut C, Lorquet S.
Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin)
in preeclampsia – a step forward but not the definitive answer.
J Reprod
Immunol
2009;
82
(2): 106–111.
17. Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral
artery remodelling: lessons from the cardiovascular field.
Placenta
2010;
31
(6): 465–474.
18. Moffett A, Colucci F. Uterine NK cells: active regulators at the mater-
nal–fetal interface. J C
lin Invest
2014;
124
(5): 1872–1879.
19. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal
inflammatory response to pregnancy.
Am J Obstet Gynecol
1999;
180
(2
Pt 1): 499–506.
uNKC
Placental ischaemia
Defective spiral
artery remodelling
Cytotrophoblasts Genetic factors
↑
sFlt-1
Endothelial
dysfunction
Glomerular
endotheliosis
Hypoxia–reperfusion
damage of
trophoblasts
HYPERTENSION,
PROTEINURIA,
OEDEMA
Pulsatile/
intermittent
blood flow
to placenta
STMBs, cytokines,
AT-1AA, imbalance
in angiogenic–anti-
angiogenic factors in
maternal circulation
↓
VEGF, PlGF
PRE-ECLAMPSIA
Fig. 1.
Aspects of pathophysiology of pre-eclampsia. VEGF:
vascular endothelial growth factor; PlGF: placental
growth factor; sFlt-1: soluble film-like tyrosine kinase.

















