
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 1, January/February 2018
AFRICA
53
activation of a number of signal-transduction pathways when
there is chronic myocyte stretch.
28,29,33,35-37
It does this by changes
in the expression of various genes involved in adaptation to
the increased load and, ultimately, to the activation of various
maladaptive pathways.
38-40
Titin complexes with a number of potential ‘signalosomes’
(a mechanosensative signalling complex), including the Z-disc-
localised protein MLP (muscle LIM protein), which has been
shown to be responsible for hypertrophy and cardiomyopathy
in MLP-deficient animals.
41,42
MLP, aside from its structural
role in the Z-disk and its interaction with signal transduction
proteins, is able to translocate to the nucleus and thereby act
as a transcription factor modifying gene expression, depending
on mechanical stretch.
43
MLP may be responsible for control
of other transcription factors coordinating alterations in the
expression of genes responsible for ventricular remodelling.
Another important titin signalosome that controls muscle gene
expression is the sarcomere M-band-associated protein titin
kinase (TK), which is activated by myocyte stretch.
38,40
TK may
primarily respond to diastolic stretch,
29
which is particularly
relevant in the case of pathological volume overload caused by
chronic MR (Fig. 2).
Pathological volume overload-induced mechanical stretch has
a number of other effects on the cardiomyocyte. For example,
in
in vitro
44
and
in vivo
45
rat experiments, TNF-
α
is produced
by cardiomyocytes, resulting in an inflammatory response to
stretch, suggesting that TNF-
α
is an important component in
the pathophysiological response of the myocardium to volume
overload. Mechanical stretch also results in the local production
of angiotensin II
46
and ROS,
47
which, via transcription factors,
such as TRAIL (TNF-related apoptosis-inducing ligand) and
NF
κ
B,
47
result in local increases in pro-inflammatory cytokines,
further contributing to activation of remodelling signal-
transduction pathways.
48,49
Finally, as discussed in more detail
below, mechanical stretch is transmitted through the ECM to
cardiomyocyte integrins, which trigger a number of intracellular
signal-transduction pathways involved in hypertrophy and
apoptosis.
31,39
Chronic primary MR increases cardiac reactive
oxidative stress
ROS play an important role in signal transduction and
physiological regulation in vascular and myocardial cells.
However, under pathological conditions, such as excessive
myocyte stretch or excessive inflammatory signals, ROS have been
shown to activate maladaptive remodelling signal-transduction
pathways.
50,51
These signal-transduction pathways include (but
are not limited to) protein phosphorylation pathways leading
to cell growth or apoptosis (depending on ROS levels and other
factors); matrix metalloproteinase activation;
52
cell cycle protein
pathways leading to apoptosis; and pathways leading to the
activation of inflammatory transcription factors such as NF
κ
B.
53
ROS are increased in patients with congestive heart failure,
54
and there is evidence of pathological increases in ROS in patients
with chronic isolated MR who still have left ventricular ejection
fractions (LVEF) above 60%.
55
These data suggest that there is an
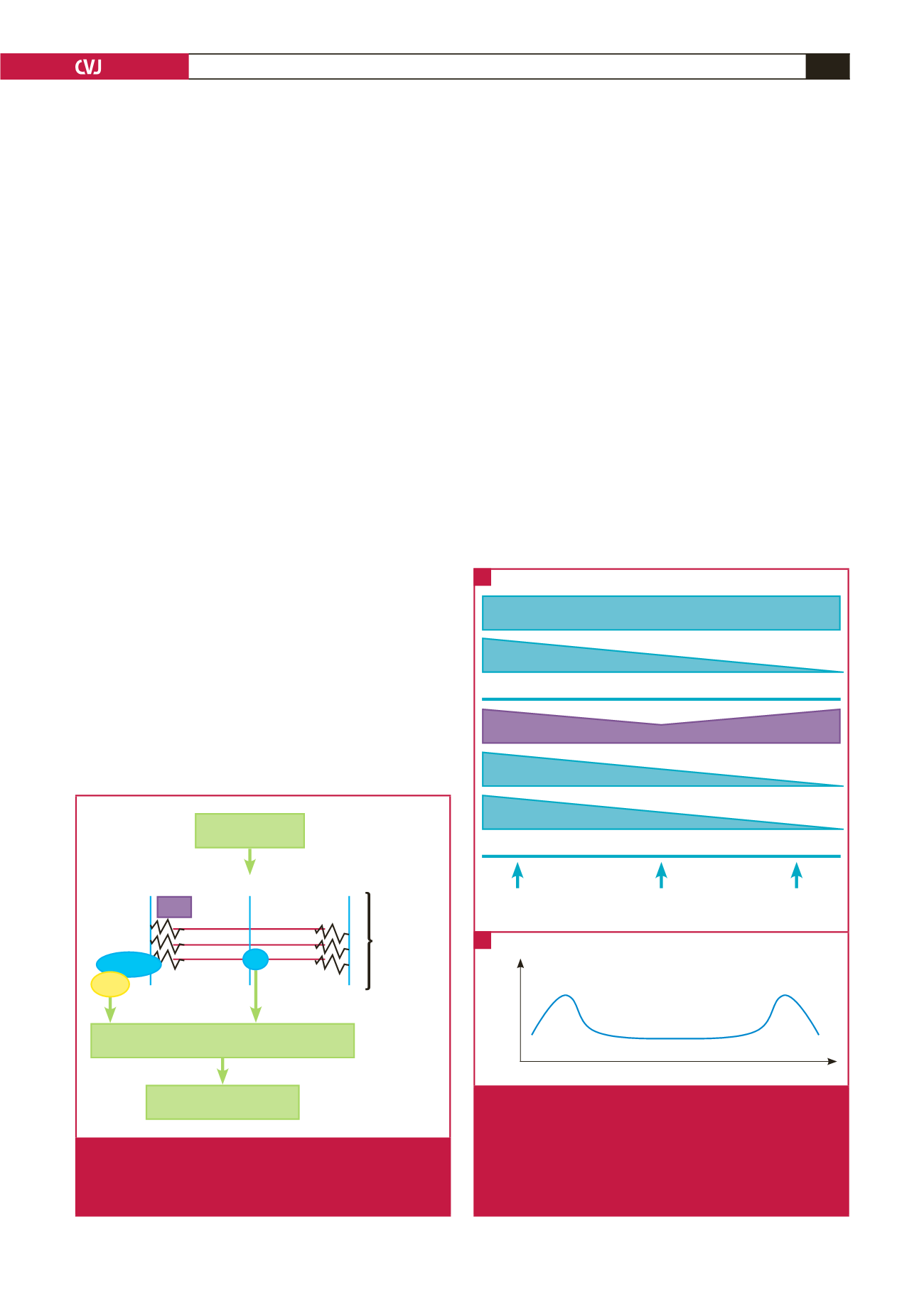
Myocyte stretch
Ventricular remodelling
Regulation of gene expression
M-band
Z-disc
Sarcomere
Titin
TK
Telethonin
MLP
Fig. 2.
Schematic representation of gene regulation in
response to myocyte sarcomere stretch via signal
transduction through MLP and TK. MLP
=
muscle LIM
protein; TK
=
titin kinase. See text for details.
Mast cells increase early and continue to increase
Inflammation
MMP activity
Interstitial collagen
β
-AR responsiveness
Adrenergic: activated throughout
ROS: increased oxidative stress throughout
Acute MR
inflammation
Decompensation
‘Compensated’
Laplace criteria met but
ongoing myocyte stress
Time
Acute MR
Decompensation
Activity of
regulatory pathways
Fig. 3.
A. Proposed time-dependent changes in various
remodelling pathways including changes in measured
prevalence of mast cells. B. Proposed overall time-
dependent changes in remodelling pathway activa-
tion. MMP
=
matrix metalloproteinases;
β
-AR
=
β
-adrenergic; ROS
=
reactive oxidative species. See
text for details.
A
B

















