
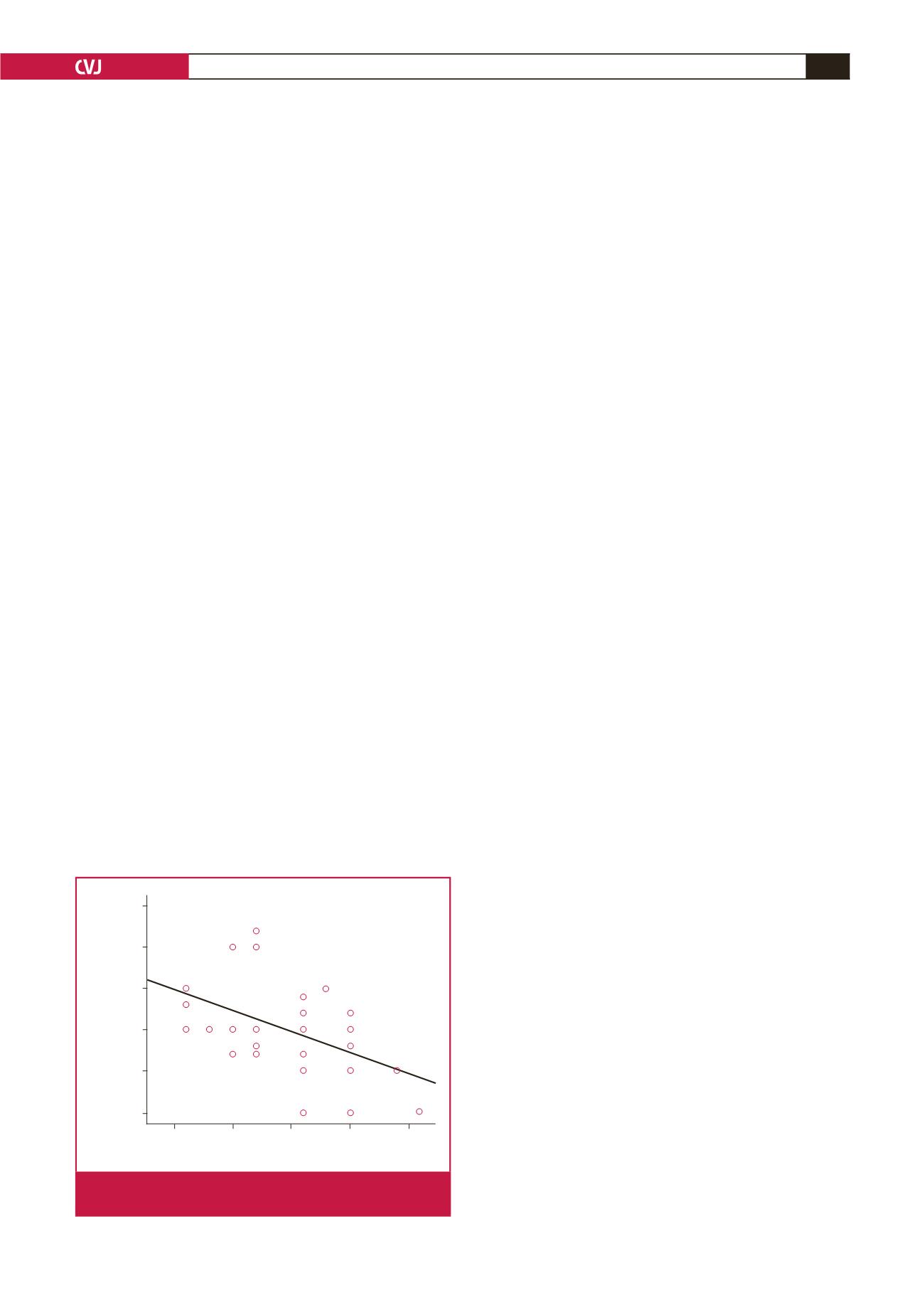
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 3, May/June 2019
AFRICA
155
imperfection and that irreversible pulmonary vascular changes
may occur at under two years of age. This is most likely the after-
effect of the high-pressure pulsatile stream transmitted from the
aorta to the pulmonary artery throughout the cardiovascular
cycle in patients with PDA.
In our study, we found that PAP was significantly higher in
children with PDA prior to closure (
p
<
0.05) in both groups.
After closure, the PAP was diminished in both groups (
p
<
0.05)
and approaching non-significance compared with the control
group (
p
>
0.05) (Tables 3, 4). Additionally, we discovered a
significant positive relationship between ASI and PAP (
r
=
0.6,
p
<
0.05) (Fig. 2).
We also found in this study that the PAP was significantly
higher in patients in group B than in those in group A preceding
closure (
p
<
0.05). After closure, there was no significant
difference between groups with regard to PAP (
p
=
0.2) (Table 2).
Note that a small number of patients with PDA and PAH and
marginal haemodynamic instability can deteriorate after PDA
closure because of non-regression of pulmonary hypertension,
progressive PVD and right heart failure. Therefore their normal
history becomes similar to that of primary or idiopathic PAH.
These patients have a better normal history if the PDA is left
untreated.
A safe examination to distinguish who may benefit from
PDA closure with long-term regression of PAH and who may
be plagued by progressive pulmonary vascular disease and right
heart failure is presently not available. Future research on the type
and degree of morphological changes in the pulmonary vessels,
individual inconsistence, and hereditary and epigenetic variables
may provide some insight into this disturbing problem. Until
such time, in clinical practice, infrequently there may be a patient
who will not benefit from closure of an expansive PDA and may
have an adverse outcome if PDA closure is attempted; however
there may be many others who have favourable outcomes.
Aortic stiffening prompts faster pulse wave velocity, and
therefore a prior heartbeat wave reflection may occur, bringing
about an expansion in focal systolic blood pressure (SBP)
and a diminishing diastolic blood pressure (DBP) with an
increment in pulse pressure. An expanded SBP may build the LV
afterload, with an expansion in myocardial oxygen demand, LV
hypertrophy, fibrosis, and inevitably, a reduction in the LVEF.
19
In our study, we found a significant negative correlation between
the ASI and LVEF prior to PDA closure (
r
=
0.66,
p
<
0.05)
(Fig. 4) and a significant positive relationship between ASI and
LVEDD prior to closure (
r
=
0.58,
p
<
0.05) (Fig. 3).
Conclusion
Aortic stiffness is increased in patients with PDA, even in cases
of small-sized PDAs and is related to weakness in heart function,
especially if PDA closure is deferred to after the age of one year.
After device closure, the ASI diminished significantly and was
associated with a notable change in heart capacity and functional
class months after device closure. The ASI may be helpful in
assessing the course of patients with PDA prior to and after
intervention. We recommend early closure of PDA, even in cases
of small-sized PDAs, and use of the ASI as a tool for following
patients with PDA before and after closure.
References
1.
Samanek M. Children with congenital heart disease: probability of
natural survival.
Pediatr Cardiol
1992;
13
(3): 152–158. DOI: 10.1007/
bf00793947.
2.
Gkaliagkousi E, Douma S. The pathogenesis of arterial stiffness and its
prognostic value in essential hypertension and cardiovascular diseases.
Hippokratia
2009;
13
(2): 70–75. PMID: 19561773.
3.
Mahfouz RA, Dewedar A, Abdelmoneim A, Hossien EM. Aortic and
pulmonary artery stiffness and cardiac function in children at risk for
obesity.
Echocardiography
2012;
29
(8): 984–990. DOI: 10.1111/j.1540-
8175.2012.01736.x.
4.
Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA.
Assay of brain natriuretic peptide (BNP) in human plasma: evidence
for high molecular weight BNP as a major plasma component in heart
failure.
J Clin Endocrinol Metab
1993;
76
(4): 832–838. DOI: 10.1210/
jcem.76.4.8473392.
5.
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera
JA,
et al
. Guidelines for the diagnosis and treatment of pulmonary
hypertension: the Task Force for the Diagnosis and Treatment of
Pulmonary Hypertension of the European Society of Cardiology
(ESC) and the European Respiratory Society (ERS), endorsed by the
International Society of Heart and Lung Transplantation (ISHLT).
Eur
Heart J
2009;
30
(20): 2493–2537. DOI: 10.1093/eurheartj/ehp297.
6.
Yang SW, Zhou YJ, Hu DY, Liu YY, Shi DM, Guo YH,
et al.
Feasibility
and safety of transcatheter intervention for complex patent ductus arte-
riosus.
Angiology
2010;
61
(4):372–376.DOI:10.1177/0003319709351874.
7.
Jekell A, Malmqvist K, Wallen NH, Mortsell D, Kahan T. Markers of
inflammation, endothelial activation, and arterial stiffness in hyperten-
sive heart disease and the effects of treatment: results from the SILVHIA
study.
J Cardiovasc Pharmacol
2013;
62
(6): 559–566. DOI: 10.1097/
fjc.0000000000000017.
8.
Park S, Lakatta EG. Role of inflammation in the pathogenesis of
arterial stiffness.
Yonsei Med J
2012;
53
(2): 258–261. DOI:10.3349/
ymj.2012.53.2.258.
9.
Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima
JA,
et al
. Relation of aortic wall thickness and distensibility to cardio-
vascular risk factors (from the Multi-Ethnic Study of Atherosclerosis
[MESA]).
Am J Cardiol
2008;
102
(4): 491–496. DOI: 10.1016/j.
amjcard.2008.04.010.
ASI
2.5
5.0
7.5
10.0
12.5
LVEF
75
70
65
60
55
50
r
= 0.575
p
= 0.001**
Fig. 4.
A significant negative correlation is shown between
the ASI and LVEF.

















