
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 31, No 1, January/February 2020
AFRICA
37
of four years nine months and four years seven months after
percutaneous PDA closure.
The duration of the follow up with regard to our cohort ranged
from 15 months to two years (median: two years). Of note, there
was no device-induced coarctation of the aorta, left pulmonary
artery stenosis, procedure-induced aortic regurgitation or left
ventricular dysfunction. Forty-nine patients had already been
discharged from follow up at the time of writing this article, as
they had completed the two-year follow-up duration as per the
study protocol.
Discussion
Morbidity and mortality associated with the PDA in preterm
infants is well documented.
6,7
In this group, failed medical
therapy with ibuprofen is reported to be around 22–24%, which
is comparable to failed treatment with oral paracetamol, at
18–31%.
36
Therefore this group was subjected to percutaneous
closure of the PDA in our unit.
Both medical treatment and surgery are fraught with
complications. Devices other than the ADO II AS are also being
explored and have been successfully utilised in percutaneous
closure of the PDA in this challenging group with lower body
weight. These include the Amplatzer vascular plug and the
Medtronic micro vascular plug.
17,37-39
Challenges faced by this
lower-weight group include, among others, difficult vascular
access, vascular access injury, excessive bleeding in relation to
body mass index, haemodynamic instability, metabolic acidosis,
hypothermia and death.
40
There have been attempts to close PDAs percutaneously using
echocardiography in the neonatal unit in preterm infants who
have haemodynamically significant PDAs.
18,19
This approach was
prompted by listed poor outcomes experienced in the cardiac
catheterisation of small infants in the catheterisation laboratory.
However, this bedside technique has not been translated into
routine clinical practice.
In our study, there was successful closure of the PDA in 57
patients (96.6%). This is comparable with the results reported
by Kang
et al
. in their multicentre study of 408 lower-weight
patients,
18
where there was also a 95% closure rate recorded in
the last recorded patient follow up. Kenny
et al
. reported on
the successful ductal closure in 16 of 17 patients, and there was
only one embolisation in this cohort.
19
The device was surgically
retrieved with the ligation of the duct. Of note, nine patients
weighed less than 6 kg, and the smallest patient was 1.7 kg in
this cohort.
In another study, the ADO II AS device was used in 60
patients to close the PDA, and 26 of these patients weighed
less than 6 kg.
22
Moreover, there was successful PDA closure in
96.6% of patients without major complications, except for one
embolisation.
Recently, the Medtronic micro vascular plug has been used to
close a PDA in eight patients weighing less than 6 kg.
37
In fact,
half of the patients in this recent study were infants weighing
less than 2.5 kg. Furthermore, there was one embolisation in this
cohort. The embolised device was retrieved transcutaneously and
an Amplatzer type II device was successfully deployed to close
the PDA.
Although, there is little data on PDA closure in premature
infants weighing less than 1 000 g, the Amplatzer vascular plug
II has been successfully used for ductal closure in this group.
38
Moreover, in our cohort, we had one preterm infant weighing less
than 1 000 g (900 g) at the time of ductal closure. In this patient,
the PDA measured 3.4 mm in diameter and was 9.3 mm long.
A 05x06L device was used to close the PDA in an anterograde
approach. The patient was discharged without any complications.
The ducts in preterm infants are usually large and tubular and
need either medical or surgical intervention.
41
All the patients in
the study cohort had ducts that were long and the majority of
these PDAs were longer than the recommended maximum length
for closure using the ADO II AS. Despite this, owing to the shape
of the device and the small diameter of the retention disks, it was
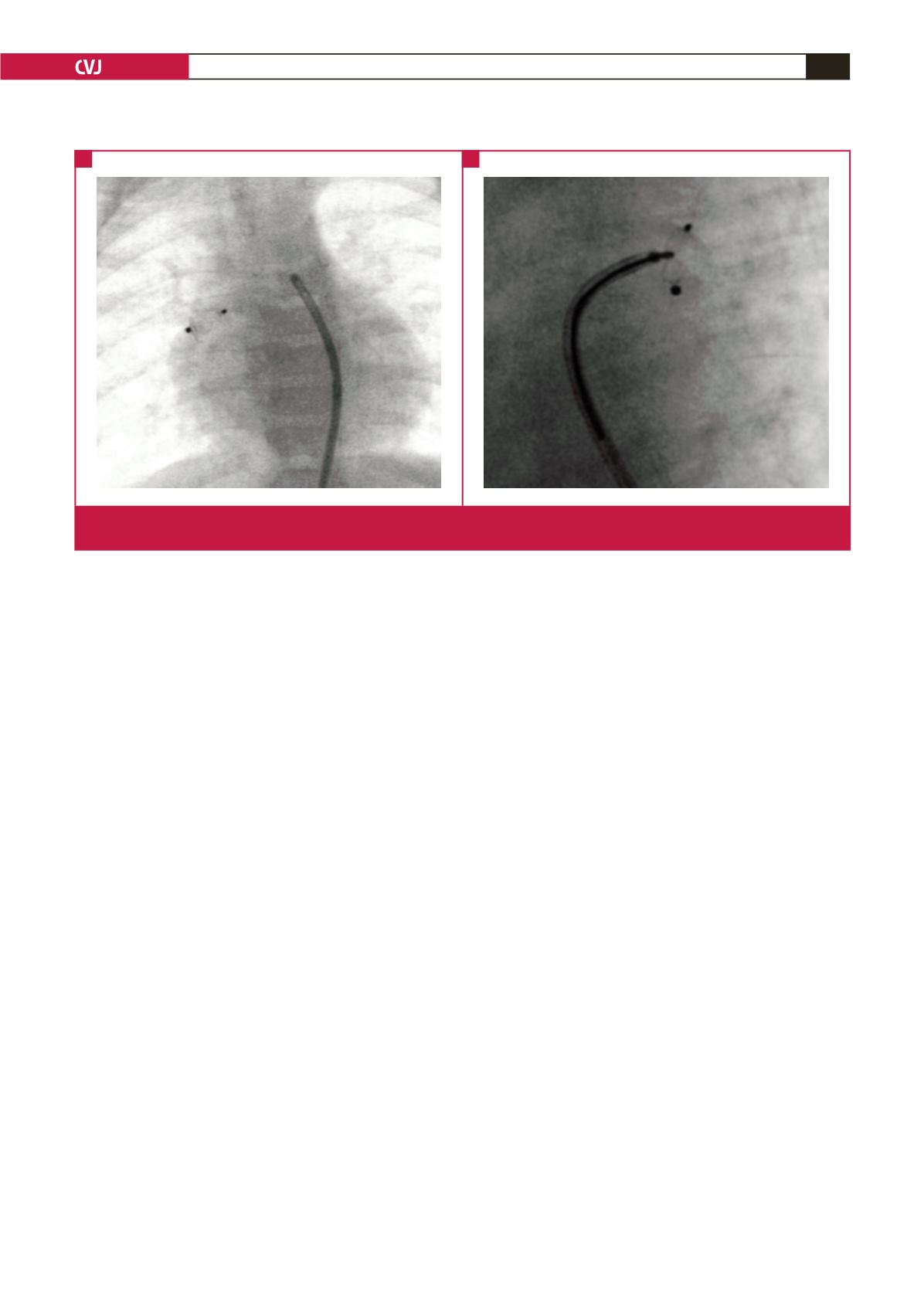
Fig. 3.
Embolised device into the right pulmonary artery (A). The device is being retrieved using a 10-mm (loop) AndraTec Exeter
snare (AndraTec GmbH, Simmernerstr, Koblenz, Germany) (B).
A
B



















