
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 2, March/April 2015
AFRICA
99
tachyarrhythmias or conduction abnormalities accounts for
25–65% of cardiac sarcoidosis-related deaths.
8
Complicated
sarcoidosis has been associated with postpartum maternal
death.
9
Our two patients delivered by caesarean section and had
no further complications.
Diagnostic tools
There are no currently accepted international guidelines for
the diagnosis of cardiac sarcoidosis. The diagnosis of cardiac
sarcoidosis can be difficult, as it can be asymptomatic or
non-specific. Cardiac sarcoidosis is manifested clinically
as a cardiomyopathy with loss of ventricular function, or
tachyarrhythmias and bradyarrhythmias (palpitations, syncope
and sudden death).
Both our patients presented with atrial arrhythmias. The first
patient (case 1) had atrial fibrillation (more frequent and faster
heart rate than before pregnancy) and the second patient (case
2) had supraventricular tachycardia. The most common location
for granulomas and scars is the left ventricular free wall, followed
by the interventricular septum, often with involvement of the
conducting system.
Endomyocardial biopsy sample for pathological research
that is positive is hard to obtain and has a low diagnostic yield
(
<
20%) because cardiac involvement tends to be patchy, and
granulomas are more likely to be located in the left ventricle and
basal part of the ventricular septum.
10
A pathological diagnosis
of cardiac sarcoidosis was not performed in our two patients
for these reasons. Instead, we chose to perform a less-invasive
cardiac MRI. Cardiac MRI with gadolinium enhancement and
a
18
F-FDG PET scan are valuable aids in the diagnosis of cardiac
sarcoidosis.
3
Since sudden death may be the first sign of cardiac
sarcoidosis, electrophysiological studies to detect any conduction
delays or increased risk of sustained arrhythmias should be
strongly considered in all patients with suspected cardiac
sarcoidosis. Most authorities recommend placement of an
electronic pacemaker for complete heart block, and an automatic
implantable cardioverter–defibrillator for ventricular fibrillation
or tachycardia and markedly reduced left ventricular ejection
fraction.
11
Sarcoidal granulomas produce angiotensin converting
enzyme (ACE), and ACE levels are elevated in 60% of patients
with sarcoidosis. However, a serum ACE level is an insensitive
and non-specific diagnostic test and a poor therapeutic guide.
3
A recently published expert consensus statement provides
guidance for clinicians on the diagnosis and management of
arrhythmias associated with cardiac sarcoidosis.
12
Unfortunately
they do not have
recommendations for pregnant patients as there
is limited evidence for this specific patient population.
Treatment
The initial treatment of sarcoidosis is often prednisone 20–40
mg daily for six to 12 weeks, with dose reduction thereafter. As
cardiac sarcoidosis is a potentially life-threatening situation, the
initial dose is 1 mg/kg daily. Although a minimum of 12 months
of maintenance therapy is often advised to prevent relapse,
several investigators believe that treatment should be stopped as
early as six months after initiation.
13
Oral steroid therapy is usually performed to treat
atrioventricular block due to cardiac sarcoidosis. However, its
efficacy against cardiac sarcoidosis in general is only about
50%.
14,15
Although cardiac sarcoidosis is a known inflammatory
disease anddespitemore than50 years of the use of corticosteroids
for treatment, there is no proof of survival benefit from this
treatment.
16
As specific guidelines on cardiac sarcoidosis in pregnant
women do not exist, in case of occurring relevant bradycardia
or tachycardia, pacemakers and ICDs may be considered
.
Since
there is no evidence of giving immunosuppressive therapy for
pregnant women, we suggest that efforts should be made to
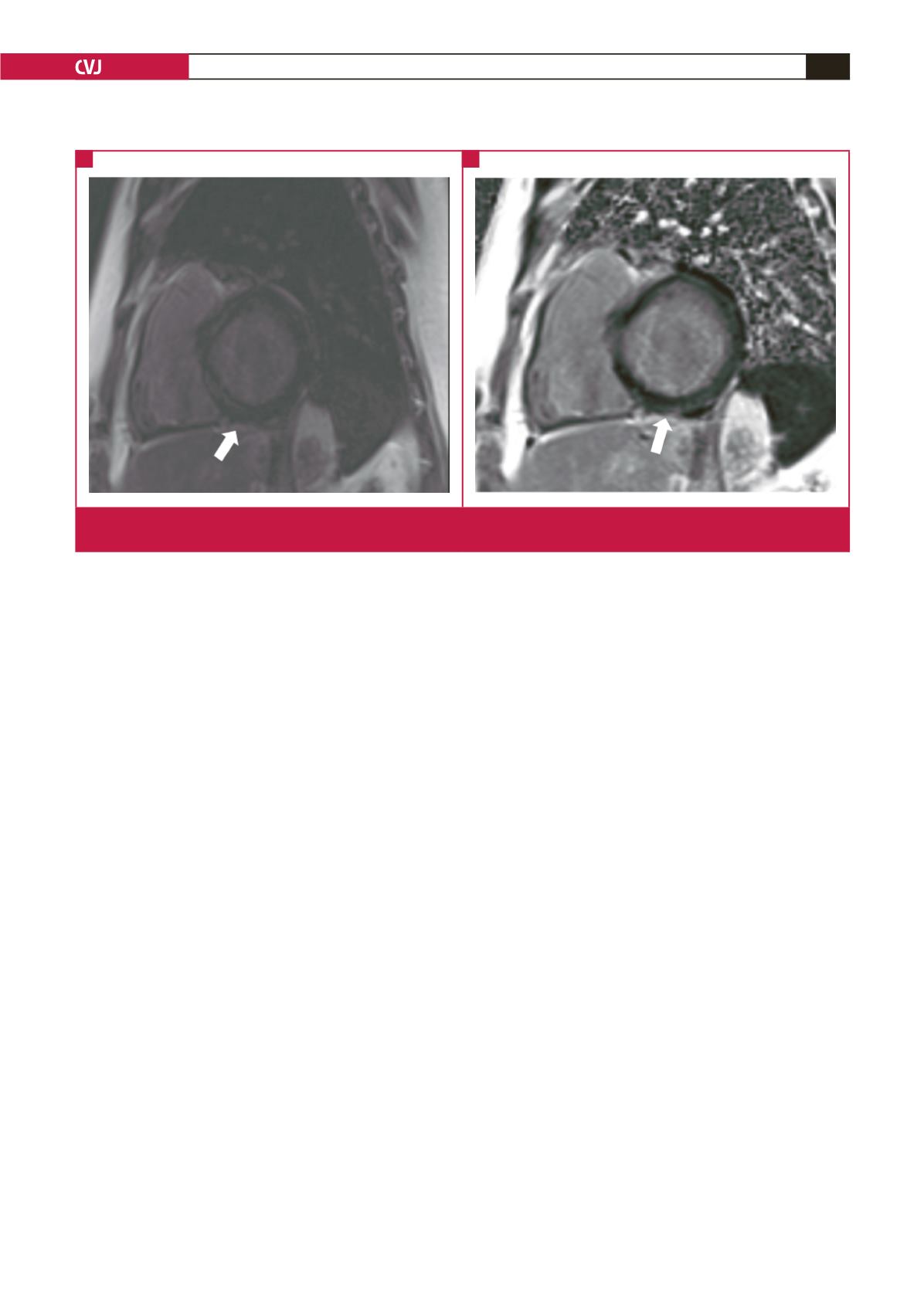
Fig. 3.
Cardiac MRI images of case 2. Basal short-axis left ventricular magnitude (A), and PSIR (B) delayed enhancement images,
demonstrating mesocardial (white arrow in A) and epicardial enhancement (white arrow in B).
A
B

















