
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 3, May/June 2015
AFRICA
e13
three times on the same day because of increased ischaemic
changes. The thrombectomy was not successful.
We suspected type 2 HIT. Heparin therapy was immediately
discontinued on postoperative day three, including all intravenous
fluids and lines. On the basis of the clinical symptoms, we used the
‘4 Ts’ clinical scoring system to test for the possibility of HIT, and
found a high probability of heparin-induced thrombocytopenia.
A laboratory test was performed on postoperative day six,
and a definitive diagnosis of HIT was made by serological test,
confirming positive antibodies to the heparin–PF4 complexes
with a slow turnaround time. Complete thrombophilic studies
were unremarkable for other hypercoagulable conditions.
Anticoagulation was immediately started with fondaparinux,
which is the only alternative anticoagulant agent in our country,
at the recommended dose for these patients (7.5 mg/day,
subcutaneous) on postoperative day four because of a fall in
platelet count of more than 50%. The patient’s platelet count had
not increased during therapy with fondaparinux after seven days.
The patient developed acute renal insufficiency requiring
haemodialysis on postoperative day nine. Fondaparinux (2.5 mg)
was also instilled directly into the dialysis circuit on dialysis days.
On the 11th day postoperatively, the patient died of multiple
organ failure despite intensive care.
Discussion
Unfractionated heparin (UFH) is routinely used worldwide
during CPB procedures and other various conditions for systemic
anticoagulation.
4
However, a small percentage of patients treated
with UFH or low-molecular weight heparin (LMWH) suffer
complications caused by side effects of the drug, the most serious
of which is HIT.
1
HIT is an adverse effect of the drug causing
potentially fatal thrombotic or thromboembolic complications.
HIT is a clinicopathological condition initiated with heparin
exposure and characterised by a fall in the platelet count and
paradoxical thrombophilia.
5,6
HIT syndrome may be classified into two distinct subtypes
based on differences in the pathophysiology and clinical features:
type 1 and type 2. Type 1 HIT is non-immune and typically
occurs as a fall in platelet count within the first two days after
starting heparin. Platelet levels generally decrease by 10–20%.
7
This condition usually resolves spontaneously without treatment
or complications within days, even with continued heparin use.
It is a non-immune-mediated disorder and appears to be due to
a direct activation of the platelets by heparin, leading to platelet
aggregation and, as a result, thrombocytopenia.
By contrast, the less common and more severe form, type
2 HIT is an immune-mediated disorder caused by antibody
formation against the circulating H-PF4 complexes. This type can
be associated with thrombotic or thromboembolic complications.
It is also known as heparin-induced thrombocytopenia and
thrombosis (HITT) and white clot syndrome due to platelet-rich
arterial thrombosis.
5
In most cases, thrombocytopenia develops
on approximately the fifth day of initiation of heparin.
8
The incidence of type 2 HIT is significantly higher after
exposure to UFH versus LMWH (2.6 vs 0.2%).
9
Surgical patients
(especially cardiac surgery) are also more likely to develop HIT
than medical patients.
5
The incidence of HITT in patients who have undergone
cardiac surgery has been estimated at between 0.12 and 1.3%.
10
Cardiac surgical patients are at a greater risk for postoperative
HITT due to several factors. First, most of these patients have
had previous exposure to heparin for diagnostic, prophylactic
and therapeutic purposes. Second, they are exposed to high-dose
intra-operative heparin during CPB, and platelet activation is
associated with surgery and CPB. Third, this exposure is usually
continued in the postoperative period (either prophylactically or
for flushing the lines).
11
The common clinical presentation of HIT involves
thrombocytopenia and thrombosis. Thrombocytopenia is the
primary manifestation of HIT, but the degree and onset of
the fall in platelet count may be variable. Type 1 HIT is often
characterised by a fall in platelet count within one and four days
after heparin exposure, with a nadir level of 100 000 cells/
μ
l,
spontaneous normalisation despite continued heparin use, and
no other clinical sequelae.
On the other hand, type 2 HIT occurs within five to 10 days
after the administration of heparin. The platelet counts fall
more significantly by
≥
50% or
≥
100 000 cells/
μ
l, with a median
nadir of ~ 60 000 cells/
μ
l.
7
However, even when platelet counts
in type 2 HIT are typically
<
20 000/
μ
l, spontaneous bleeding is
uncommon. Thrombosis is the main contributor to morbidity
and mortality associated with type 2 HIT, and HIT is fatal in
an estimated 5–10% of patients, typically due to thrombotic or
thromboembolic events.
Thrombosis may accompany thrombocytopenia in 30–60%
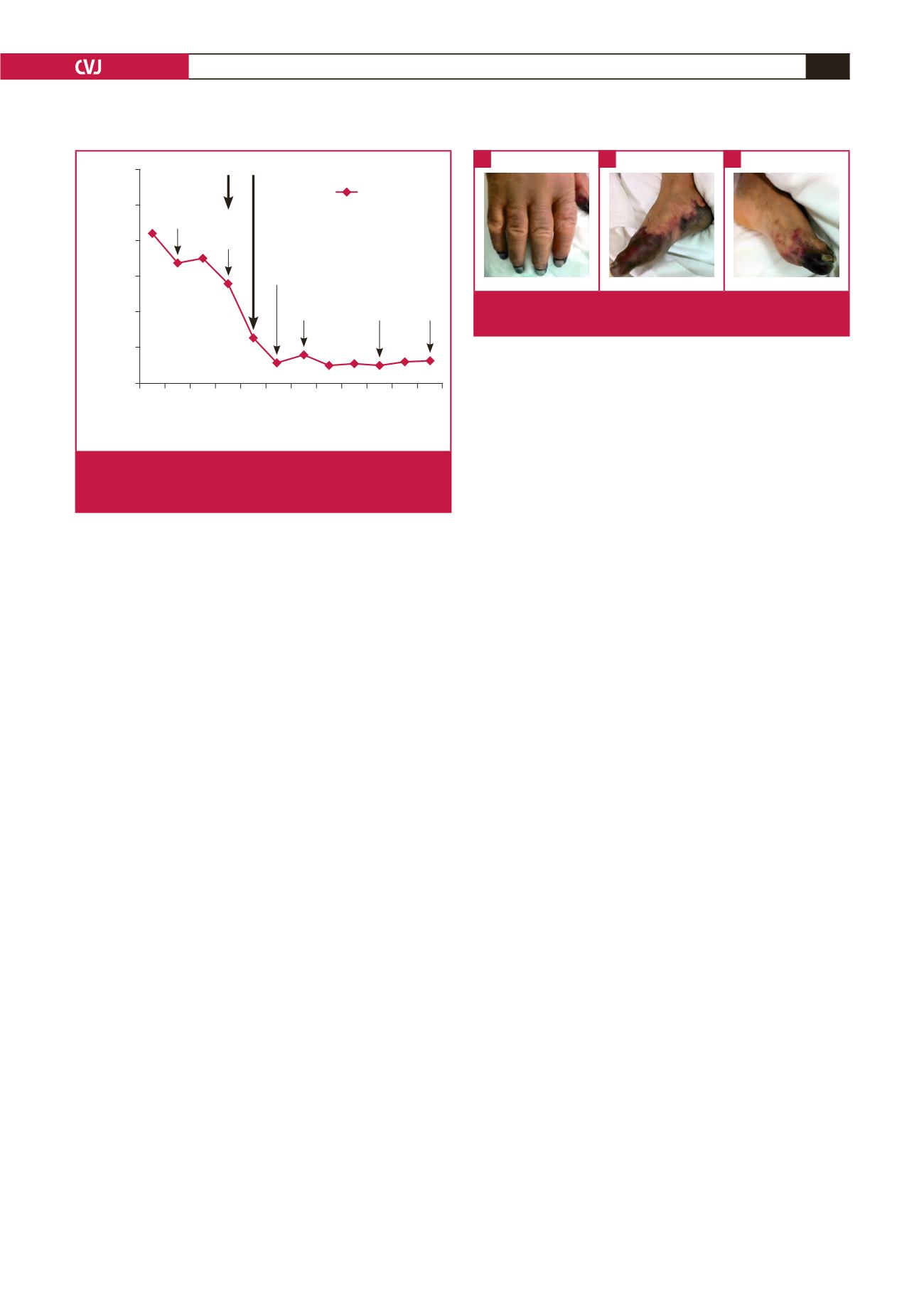
Fig. 3.
The ischaemic changes in the right hand (A), right foot
(B), and left food (C).
A
B
C
300
250
200
150
100
50
0
Pre-
op
1 2 3 4 5 6 7 8 9 10 11
Hospital day
Platelet count (x 1 000)
Platelet count
26 500 U
heparin
in OR
Died
ARF and
haemodialysis
Both food
ischaemia
Fondaparinux was started
Heparin was stopped
Weaning
time from
the IABP
Right-hand ischaemia
and brachial
thrombectomy
Fig. 2.
Platelet counts and key clinical events during hospi-
talisation. ARF: acute renal failure, IABP: intra-aortic balloon
pump, OR: operating room.

















